The treatment of severe symptomatic aortic stenosis has been revolutionized by the technique of transcatheter valve replacement. The purpose of this study was to present the outcomes and predictors of mortality in patients enrolled between 2010 and 2011 in the Transcatheter Aortic Valve Replacement National Registry.
MethodsWe collected 131 preprocedural, 31 periprocedural, and 76 follow-up variables, and analyzed the immediate implant success rate, the 30-day safety endpoint, and all-cause 30-day and mid-term (mean follow-up, 244 days) mortality.
ResultsFrom January 2010 to December 2011, a total of 1416 patients were included: 806 with Edwards valves and 610 with CoreValves. The implant success and 30-day mortality rates were 94% and 8%, respectively, without differences between types of valves and approaches. The 30-day safety endpoint and mid-term mortality rates were 14% and 16%, respectively, which were also similar between groups. The presence of comorbidities (renal failure, peripheral vascular disease, ejection fraction, and atrial fibrillation), the need for conversion to surgery, and at least moderate aortic regurgitation after transcatheter aortic valve implantation were identified as independent predictors of in-hospital and mid-term mortality.
ConclusionsThe prognosis of valve implant patients could be improved by including comorbidities in patient selection and by minimizing the degree of residual aortic regurgitation to optimize the results of the procedure.
Keywords
.
INTRODUCTIONThe treatment of severe symptomatic aortic stenosis has evolved considerably in recent years due to the development of the transcatheter aortic valve implantation (TAVI) technique. This procedure has been shown to be superior to conservative medical treatment in patients who are not surgical candidates1 and is not inferior to conventional surgery for high-risk surgical patients.2 Accordingly, in new valve disease treatment guidelines jointly drawn up by the European Society of Cardiology and the European Association for Cardio-Thoracic Surgery, 3 TAVI has been given a class I/B recommendation for nonsurgical patients and a class IIa/B recommendation for high-risk surgical patients. In addition, these guidelines stress that the decision to perform TAVI be based on consensus among a “heart team”, and that the procedure be carried out in a center with an on-site cardiac surgery team.3
In 2010, the TAVI National Committee (Comité Nacional TAVI) was formed to create a national registry of TAVI procedures carried out in Spain and to disseminate the registry results. The total number of procedures is published annually in a report by the Working Group on Cardiac Catheterization and Interventional Cardiology on the data collected by the national activity registry (registro de actividad de la Sección de Hemodinámica y Cardiología Intervencionista).4
Since the first implantation in Spain,5 reports have been published of valve implantation results in several high-volume centers, generally of a single valve type.6,7 Accordingly, there are few data on other types of valves and approaches that are currently in general use in Spain.8 Consequently, the aim of the present study was to determine the results of patients who underwent treatment with TAVI in a representative Spanish population from 2010 and to establish predictors of in-hospital and mid-term mortality.
METHODSPatient SelectionThe TAVI National Committee project is a collaboration between the Working Group on Cardiac Catheterization and Interventional Cardiology of the Spanish Society of Cardiology and the Spanish Society of Thoracic-Cardiovascular Surgery. The project has the following objectives: to determine TAVI activity and ascertain the results of these treatments in Spain, develop scientific studies based on the data accumulated in the registry, establish treatment recommendations related to TAVI procedures, and collaborate with similar international registries. The ex officio members of this committee are 3 interventional cardiologists, 3 cardiac surgeons, 1 clinical cardiologist, and a member of the Board of the Working Group on Cardiac Catheterization and Interventional Cardiology, who is in charge of the registry. This committee is renewed every 3 years. There is no specific funding for this registry, although the companies Edwards, Medtronic, and Boston Scientific have donated an unrestricted grant to the Spanish Society of Cardiology. Registry design and data publication are the exclusive responsibility of the members of the TAVI National Committee, which is free from industry body influence. The registry complies with Spanish data protection laws and has been approved by a central ethics board. Center participation in this registry is voluntary.
The individualized data of patients who underwent TAVI procedures from 2010 were collected in this registry through an electronic case report form. A total of 131 preoperative, 31 perioperative, and 76 clinical follow-up variables were collected. Of these, approximately 75% were compulsory variables.
Study VariablesIn accordance with the recommendations of the Valve Academic Research Consortium,9 the immediate implant success rate and the combined 30-day safety endpoint (all-cause mortality, major stroke, life-threatening bleeding, myocardial infarction, RIFLE grade 3 acute kidney injury, and repeat procedure for valve-related dysfunction) were evaluated. In addition, all-cause mortality was evaluated at 30 days and at maximum follow-up.
Device DescriptionThe registry is open to any type of commercial device. To date, only 2 types of valves have been included: the Medtronic CoreValve™ and Edwards SAPIEN™. The latter can be implanted via a femoral, transapical, or transaortic approach, whereas the former can be implanted though a femoral, axillary/subclavian, or transaortic approach. The technical specifications of these devices have previously been described.10
Statistical AnalysisContinuous and categorical variables are presented as mean (standard deviation) and as frequencies and percentages, respectively. Continuous variables were compared with Student's t-test or analysis of variance, whereas categorical variables were compared with a chi-square or Fisher's exact test, as appropriate. A logistic regression model was used to identify predictors of hospital mortality by introducing variables with P<.05 in the univariate analysis (age, peripheral vascular disease, creatinine clearance, EuroSCORE, and peak gradient) and clinically relevant variables (type of valve used and apical approach or not). The backward stepwise method was used. The results are expressed as odds ratios and their 95% confidence intervals (95%CIs). Multivariate analysis for the prediction of mortality during follow-up was performed through Cox regression by including all variables that were significant in the univariate analysis (sex, left ventricular ejection fraction, EuroSCORE, implant device failure, and conversion to surgery or aortic regurgitation after implantation) and those considered clinically relevant (type of valve used and apical approach or not). The results are expressed in hazard ratios and their 95%CIs. The survival curve was obtained with the Kaplan-Meier method. A Breslow test was used to compare survival between groups with the mortality variable during follow-up. The statistical analysis was performed with SPSS 15.0 software for Windows (SPSS Inc.; Chicago, Illinois, United States). To determine the representativeness of the TAVI national registry, the ratio between the numbers of patients included in the registry was compared with the number of valves sold during the recruitment period.
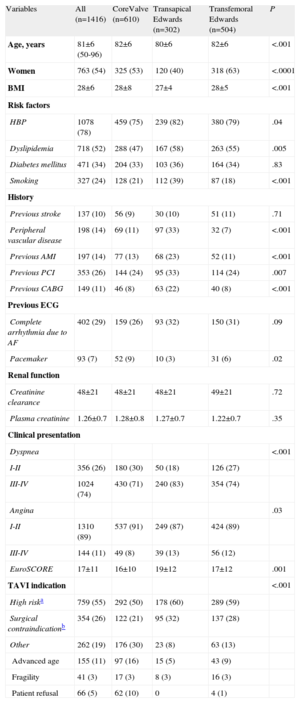
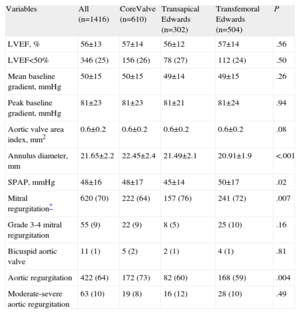
RESULTSBaseline CharacteristicsFrom January 2010 to the end of December 2011, 1416 patients were included in the TAVI registry, representing 80% of the total number of valves (1767) implanted in Spain during that year (according to data obtained from the 2 transcatheter prosthesis companies: Medtronic Ibérica SA, Madrid, and Edwards Lifesciences España SL, Paterna, Valencia). Of these 1416 patients, 806 received an Edwards valve (80% of the total number of valves sold) and 610 received a Medtronic CoreValve (81% of the total sold). By approach and number of implanted valves, a higher percentage of valves were inserted through the transfemoral approach than through the transapical approach: 82% (1114/1359) vs 74% (302/408) (P<.0001). The baseline characteristics of the population as a whole and stratified by approach and type of valve are shown in Table 1. The patients who underwent transapical prosthesis implantation were significantly younger, with a lower percentage of women, and showed a higher frequency of peripheral vascular disease, previous myocardial infarction, and previous percutaneous or surgical revascularization. In general, the EuroSCORE in this group of patients was significantly higher than that in the groups who underwent transfemoral implantation of Edwards or CoreValve devices. Surgical contraindication1 and high risk (defined by a EuroSCORE>152) were clinical indications in about 90% of patients treated with an Edwards valve and in 70% of those treated with a CoreValve (Table 1). The baseline ecocardiograph data are presented in Table 2. There were no clinically relevant differences between the types of valves and approaches, except for a significantly larger annulus size in patients treated with a CoreValve.
Baseline Characteristics by Population and by Type of Valve and Approach
| Variables | All (n=1416) | CoreValve (n=610) | Transapical Edwards (n=302) | Transfemoral Edwards (n=504) | P |
| Age, years | 81±6 (50-96) | 82±6 | 80±6 | 82±6 | <.001 |
| Women | 763 (54) | 325 (53) | 120 (40) | 318 (63) | <.0001 |
| BMI | 28±6 | 28±8 | 27±4 | 28±5 | <.001 |
| Risk factors | |||||
| HBP | 1078 (78) | 459 (75) | 239 (82) | 380 (79) | .04 |
| Dyslipidemia | 718 (52) | 288 (47) | 167 (58) | 263 (55) | .005 |
| Diabetes mellitus | 471 (34) | 204 (33) | 103 (36) | 164 (34) | .83 |
| Smoking | 327 (24) | 128 (21) | 112 (39) | 87 (18) | <.001 |
| History | |||||
| Previous stroke | 137 (10) | 56 (9) | 30 (10) | 51 (11) | .71 |
| Peripheral vascular disease | 198 (14) | 69 (11) | 97 (33) | 32 (7) | <.001 |
| Previous AMI | 197 (14) | 77 (13) | 68 (23) | 52 (11) | <.001 |
| Previous PCI | 353 (26) | 144 (24) | 95 (33) | 114 (24) | .007 |
| Previous CABG | 149 (11) | 46 (8) | 63 (22) | 40 (8) | <.001 |
| Previous ECG | |||||
| Complete arrhythmia due to AF | 402 (29) | 159 (26) | 93 (32) | 150 (31) | .09 |
| Pacemaker | 93 (7) | 52 (9) | 10 (3) | 31 (6) | .02 |
| Renal function | |||||
| Creatinine clearance | 48±21 | 48±21 | 48±21 | 49±21 | .72 |
| Plasma creatinine | 1.26±0.7 | 1.28±0.8 | 1.27±0.7 | 1.22±0.7 | .35 |
| Clinical presentation | |||||
| Dyspnea | <.001 | ||||
| I-II | 356 (26) | 180 (30) | 50 (18) | 126 (27) | |
| III-IV | 1024 (74) | 430 (71) | 240 (83) | 354 (74) | |
| Angina | .03 | ||||
| I-II | 1310 (89) | 537 (91) | 249 (87) | 424 (89) | |
| III-IV | 144 (11) | 49 (8) | 39 (13) | 56 (12) | |
| EuroSCORE | 17±11 | 16±10 | 19±12 | 17±12 | .001 |
| TAVI indication | <.001 | ||||
| High riska | 759 (55) | 292 (50) | 178 (60) | 289 (59) | |
| Surgical contraindicationb | 354 (26) | 122 (21) | 95 (32) | 137 (28) | |
| Other | 262 (19) | 176 (30) | 23 (8) | 63 (13) | |
| Advanced age | 155 (11) | 97 (16) | 15 (5) | 43 (9) | |
| Fragility | 41 (3) | 17 (3) | 8 (3) | 16 (3) | |
| Patient refusal | 66 (5) | 62 (10) | 0 | 4 (1) | |
AF, atrial fibrillation; AMI, acute myocardial infarction; BMI, body mass index; CABG, coronary artery bypass grafting; ECG, electrocardiogram; HBP, high blood pressure; PCI, percutaneous coronary intervention; TAVI, transcatheter aortic valve implantation.
Values are expressed as no. (%) or mean±standard deviation (range).
Baseline Echocardiograph Characteristics by Population and by Type of Valve and Approach
| Variables | All (n=1416) | CoreValve (n=610) | Transapical Edwards (n=302) | Transfemoral Edwards (n=504) | P |
| LVEF, % | 56±13 | 57±14 | 56±12 | 57±14 | .56 |
| LVEF<50% | 346 (25) | 156 (26) | 78 (27) | 112 (24) | .50 |
| Mean baseline gradient, mmHg | 50±15 | 50±15 | 49±14 | 49±15 | .26 |
| Peak baseline gradient, mmHg | 81±23 | 81±23 | 81±21 | 81±24 | .94 |
| Aortic valve area index, mm2 | 0.6±0.2 | 0.6±0.2 | 0.6±0.2 | 0.6±0.2 | .08 |
| Annulus diameter, mm | 21.65±2.2 | 22.45±2.4 | 21.49±2.1 | 20.91±1.9 | <.001 |
| SPAP, mmHg | 48±16 | 48±17 | 45±14 | 50±17 | .02 |
| Mitral regurgitation* | 620 (70) | 222 (64) | 157 (76) | 241 (72) | .007 |
| Grade 3-4 mitral regurgitation | 55 (9) | 22 (9) | 8 (5) | 25 (10) | .16 |
| Bicuspid aortic valve | 11 (1) | 5 (2) | 2 (1) | 4 (1) | .81 |
| Aortic regurgitation | 422 (64) | 172 (73) | 82 (60) | 168 (59) | .004 |
| Moderate-severe aortic regurgitation | 63 (10) | 19 (8) | 16 (12) | 28 (10) | .49 |
LVEF, left ventricular ejection fraction; SPAP, systolic pulmonary arterial pressure.
Values are expressed as no. (%) or mean±standard deviation.
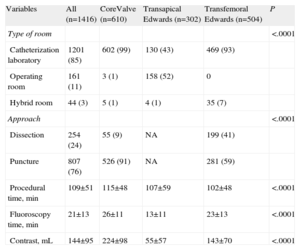
Procedure data are presented in Table 3. The procedure was mostly performed in the catheterization laboratory in patients undergoing the transfemoral approach, and was largely equally divided between the cardiovascular operating room and the catheterization laboratory in patients undergoing the transapical approach. A hybrid operating room was only available for 3% of the procedures. For procedures with a transfemoral approach, the puncture method—rather than femoral dissection—was more common (76% of the overall population; 91% of the CoreValve patients vs 59% of Edwards patients; P<.0001). Among patients receiving a CoreValve, the size of the device was 26mm in 61%, 29mm in 39%, and 31mm in 0.3%. For patients receiving an Edwards device, the size was 23 mm in 54%, 26 mm in 43%, 29mm in 3.4%, and 20mm in 0.3%. The procedure time, fluoroscopy time, and contrast volume were significantly higher in the CoreValve procedures.
Procedure Data by Population and by Type of Valve and Approach
| Variables | All (n=1416) | CoreValve (n=610) | Transapical Edwards (n=302) | Transfemoral Edwards (n=504) | P |
| Type of room | <.0001 | ||||
| Catheterization laboratory | 1201 (85) | 602 (99) | 130 (43) | 469 (93) | |
| Operating room | 161 (11) | 3 (1) | 158 (52) | 0 | |
| Hybrid room | 44 (3) | 5 (1) | 4 (1) | 35 (7) | |
| Approach | <.0001 | ||||
| Dissection | 254 (24) | 55 (9) | NA | 199 (41) | |
| Puncture | 807 (76) | 526 (91) | NA | 281 (59) | |
| Procedural time, min | 109±51 | 115±48 | 107±59 | 102±48 | <.0001 |
| Fluoroscopy time, min | 21±13 | 26±11 | 13±11 | 23±13 | <.0001 |
| Contrast, mL | 144±95 | 224±98 | 55±57 | 143±70 | <.0001 |
NA, not available.
Values are expressed as no. (%) or mean±standard deviation.
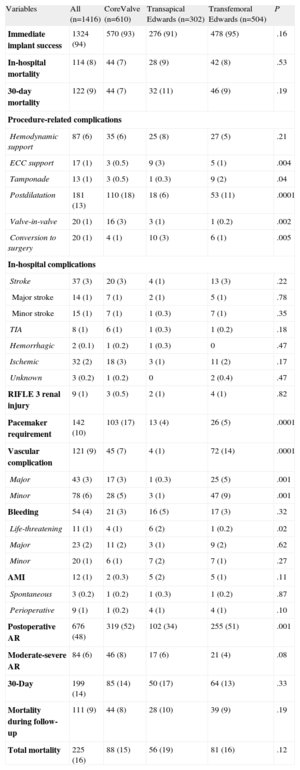
The in-hospital results are shown in Table 4. The overall immediate success rate of prosthesis implantation was 94%. By approach, the transapical success rate tended to be lower than that of the transfemoral approach (91% vs 94%; P=.09). We found a significantly greater requirement for cardiopulmonary bypass support and conversion to conventional surgery in patients receiving valves implanted via the transapical approach, a higher rate of tamponade for Edwards valves implanted via the transfemoral approach, and a greater need for postdilatation with CoreValve valves. All conversions to surgery (n=20) were to conventional valve replacement surgery, although 3 required additional aortic dissection surgery. The reasons for conversion to surgery were severe residual aortic regurgitation (n=11), poor implantation (n=4), valve migration (n=2), and aortic dissection (n=3). During hospitalization, 114 patients (8%) died, without differences due to types of valve or approach. In general, the complication rate was low. The incidences of stroke, renal failure, and myocardial infarction were similar between the valve and approach types. Notably, there was a significantly greater need for pacemakers with CoreValves, a higher rate of major and minor vascular complications with Edwards valves implanted via the transfemoral approach, and a greater overall presence of residual aortic regurgitation with valves implanted via the transfemoral approach. In addition, the prevalence of life-threatening bleeding was higher with the transapical approach. Overall, the mortality and 30-day combined safety endpoint6 rates, at 9% and 14%, respectively, were similar between the 2 types of valves and approaches.
In-hospital and Follow-up Results by Population and by Type of Valve and Approach
| Variables | All (n=1416) | CoreValve (n=610) | Transapical Edwards (n=302) | Transfemoral Edwards (n=504) | P |
| Immediate implant success | 1324 (94) | 570 (93) | 276 (91) | 478 (95) | .16 |
| In-hospital mortality | 114 (8) | 44 (7) | 28 (9) | 42 (8) | .53 |
| 30-day mortality | 122 (9) | 44 (7) | 32 (11) | 46 (9) | .19 |
| Procedure-related complications | |||||
| Hemodynamic support | 87 (6) | 35 (6) | 25 (8) | 27 (5) | .21 |
| ECC support | 17 (1) | 3 (0.5) | 9 (3) | 5 (1) | .004 |
| Tamponade | 13 (1) | 3 (0.5) | 1 (0.3) | 9 (2) | .04 |
| Postdilatation | 181 (13) | 110 (18) | 18 (6) | 53 (11) | .0001 |
| Valve-in-valve | 20 (1) | 16 (3) | 3 (1) | 1 (0.2) | .002 |
| Conversion to surgery | 20 (1) | 4 (1) | 10 (3) | 6 (1) | .005 |
| In-hospital complications | |||||
| Stroke | 37 (3) | 20 (3) | 4 (1) | 13 (3) | .22 |
| Major stroke | 14 (1) | 7 (1) | 2 (1) | 5 (1) | .78 |
| Minor stroke | 15 (1) | 7 (1) | 1 (0.3) | 7 (1) | .35 |
| TIA | 8 (1) | 6 (1) | 1 (0.3) | 1 (0.2) | .18 |
| Hemorrhagic | 2 (0.1) | 1 (0.2) | 1 (0.3) | 0 | .47 |
| Ischemic | 32 (2) | 18 (3) | 3 (1) | 11 (2) | .17 |
| Unknown | 3 (0.2) | 1 (0.2) | 0 | 2 (0.4) | .47 |
| RIFLE 3 renal injury | 9 (1) | 3 (0.5) | 2 (1) | 4 (1) | .82 |
| Pacemaker requirement | 142 (10) | 103 (17) | 13 (4) | 26 (5) | .0001 |
| Vascular complication | 121 (9) | 45 (7) | 4 (1) | 72 (14) | .0001 |
| Major | 43 (3) | 17 (3) | 1 (0.3) | 25 (5) | .001 |
| Minor | 78 (6) | 28 (5) | 3 (1) | 47 (9) | .001 |
| Bleeding | 54 (4) | 21 (3) | 16 (5) | 17 (3) | .32 |
| Life-threatening | 11 (1) | 4 (1) | 6 (2) | 1 (0.2) | .02 |
| Major | 23 (2) | 11 (2) | 3 (1) | 9 (2) | .62 |
| Minor | 20 (1) | 6 (1) | 7 (2) | 7 (1) | .27 |
| AMI | 12 (1) | 2 (0.3) | 5 (2) | 5 (1) | .11 |
| Spontaneous | 3 (0.2) | 1 (0.2) | 1 (0.3) | 1 (0.2) | .87 |
| Perioperative | 9 (1) | 1 (0.2) | 4 (1) | 4 (1) | .10 |
| Postoperative AR | 676 (48) | 319 (52) | 102 (34) | 255 (51) | .001 |
| Moderate-severe AR | 84 (6) | 46 (8) | 17 (6) | 21 (4) | .08 |
| 30-Day | 199 (14) | 85 (14) | 50 (17) | 64 (13) | .33 |
| Mortality during follow-up | 111 (9) | 44 (8) | 28 (10) | 39 (9) | .19 |
| Total mortality | 225 (16) | 88 (15) | 56 (19) | 81 (16) | .12 |
AMI, acute myocardial infarction; AR, aortic regurgitation; ECC, extracorporeal circulation; TIA, transient ischemic attack.
Hemodynamic support indicates the use of inotropes and/or an intra-aortic balloon pump.
Values are shown as no. (%).
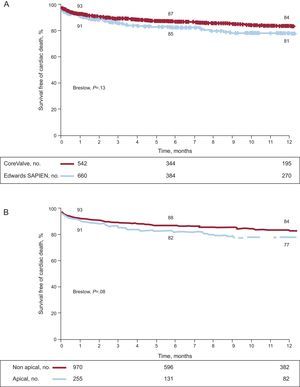
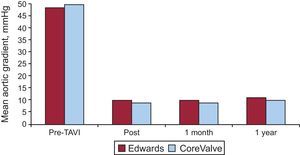
The mean clinical follow-up duration was 244 days. The reported follow-up time was shorter for valves implanted via the transapical approach than via the transfemoral approach (206 [200] vs 254 [223] days; P=.0001). The overall mortality rate following hospital discharge was 9%, which resulted in an accumulated mortality rate of 16% and which tended to be higher in patients treated via the transapical approach (Figs. 1A and B). From the clinical point of view, the percentages of patients with class III/IV dyspnea and class III/IV angina decreased from 76% and 11% before the procedure to 9% and 0.1%, respectively, during follow-up, without differences between valve or approach type. The changes in the transvalvular gradients on echocardiography during follow-up are shown in Figure 2. There were no significant differences between the types of valve or approach (Table 4).
Mean aortic gradient (mmHg) at baseline, after the procedure (post), and at the 1-month and 1-year follow-up points for CoreValve and Edwards valves. There were no significant differences between the two types of valves or significant changes in the gradients obtained after the procedure and at the 1-year follow-up. TAVI, transcatheter aortic valve implantation.
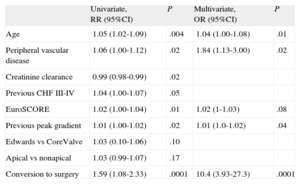
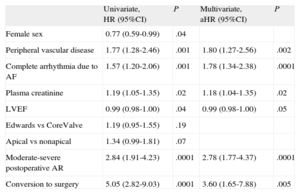
Age, peripheral vascular disease (according to the definition of the logistic EuroSCORE), conversion to surgery, and aortic stenosis severity were 30-day predictors of mortality (Table 5). At maximum follow-up, the predictors of mortality were found to be peripheral vascular disease, renal failure, atrial fibrillation, ejection fraction, conversion to surgery, and residual aortic regurgitation (Table 6). Preoperative mitral regurgitation was associated with in-hospital (odds ratio=4.12; 95%CI, 1.99-8.5; P=.001) and maximum follow-up (hazard ratio=1.67; 95%CI, 0.94-2.96; P=.09) mortality. However, preoperative mitral regurgitation could not be included in the multivariate analysis because it was recorded in only 63% of patients.
Thirty-day Predictors of Mortality
| Univariate, RR (95%CI) | P | Multivariate, OR (95%CI) | P | |
| Age | 1.05 (1.02-1.09) | .004 | 1.04 (1.00-1.08) | .01 |
| Peripheral vascular disease | 1.06 (1.00-1.12) | .02 | 1.84 (1.13-3.00) | .02 |
| Creatinine clearance | 0.99 (0.98-0.99) | .02 | ||
| Previous CHF III-IV | 1.04 (1.00-1.07) | .05 | ||
| EuroSCORE | 1.02 (1.00-1.04) | .01 | 1.02 (1-1.03) | .08 |
| Previous peak gradient | 1.01 (1.00-1.02) | .02 | 1.01 (1.0-1.02) | .04 |
| Edwards vs CoreValve | 1.03 (0.10-1.06) | .10 | ||
| Apical vs nonapical | 1.03 (0.99-1.07) | .17 | ||
| Conversion to surgery | 1.59 (1.08-2.33) | .0001 | 10.4 (3.93-27.3) | .0001 |
95%CI, 95% confidence interval; CHF, congestive heart failure; OR, odds ratio; RR, relative risk.
For quantitative variables, the risk defined by the OR is per unit of increase in the value of the variable.
Mid-term Predictors of Mortality
| Univariate, HR (95%CI) | P | Multivariate, aHR (95%CI) | P | |
| Female sex | 0.77 (0.59-0.99) | .04 | ||
| Peripheral vascular disease | 1.77 (1.28-2.46) | .001 | 1.80 (1.27-2.56) | .002 |
| Complete arrhythmia due to AF | 1.57 (1.20-2.06) | .001 | 1.78 (1.34-2.38) | .0001 |
| Plasma creatinine | 1.19 (1.05-1.35) | .02 | 1.18 (1.04-1.35) | .02 |
| LVEF | 0.99 (0.98-1.00) | .04 | 0.99 (0.98-1.00) | .05 |
| Edwards vs CoreValve | 1.19 (0.95-1.55) | .19 | ||
| Apical vs nonapical | 1.34 (0.99-1.81) | .07 | ||
| Moderate-severe postoperative AR | 2.84 (1.91-4.23) | .0001 | 2.78 (1.77-4.37) | .0001 |
| Conversion to surgery | 5.05 (2.82-9.03) | .0001 | 3.60 (1.65-7.88) | .005 |
95%CI, 95% confidence interval; AF, atrial fibrillation; aHR, adjusted hazard ratio; AR, aortic regurgitation; HR, hazard ratio; LVEF, left ventricular ejection fraction.
For quantitative variables, the risk defined by the HR is per unit of increase in the value of the variable.
This multicenter national registry describes the results of an extensive cohort of patients who underwent a TAVI procedure. This registry has been able to include 80% of all transcatheter valves implanted in Spain between 2010 and 2011. The 30-day (8%) and maximum follow-up (16%) mortality rates are similar to those described in other registries, such as the Canadian (10.4% at 30 days and 22.1% at 8 months),11 the French (FRANCE-2; 9.7% at 30 days and 24% at 1 year),12 the British (7.1% at 30 days and 26.3% at 2 years),13 and the Italian (5.4% at 30 days and 15% at 1 year)14 registries.
The characteristics of the patients were differentiated by the type of approach used, with the transapical approach showing more comorbidities and a higher EuroSCORE than the transfemoral approach. However, after correcting for confounding variables through multivariate analysis, there were no differences in mortality. Once the hospitalization phase was over, no differences were seen in the durability of the valve or long-term mortality by type of valve or approach.
The most important predictive factors of mortality were conversion to conventional surgery, comorbidities, and aortic regurgitation=3 after the procedure. The need to convert to conventional surgery is a reflection of the complexity of the procedure and the appearance of periprocedural complications, which undoubtedly lead to higher mortality. The comorbidities that we found to be associated with mortality were peripheral vascular disease, atrial fibrillation, and renal failure.
Peripheral vascular disease is frequently encountered in patients treated with TAVI. In addition, this variable increases the EuroSCORE. In a German registry, patients with peripheral vascular disease showed higher levels of vascular complications, myocardial infarction, renal failure, and mortality.15 Nonetheless, the use of a transapical approach in these patients was not associated with a reduction in the total risk associated with peripheral vascular disease.15
Atrial fibrillation and renal failure have previously been associated with a worse prognosis following TAVI in a multicenter registry of 339 patients.16 Chronic atrial fibrillation has also been associated with a worse prognosis following cardiac surgery.17 Patients with atrial fibrillation can die of noncardiac (eg, fatal bleeding) or vascular (eg, stroke) causes. In this subgroup of patients, strict control of anticoagulant therapy and avoiding its combination with other antithrombotic therapies are essential for a good long-term prognosis. Although the post-TAVI kidney injury rate was low in our registry, baseline renal function was associated with mortality during follow-up. In various series, baseline renal dysfunction appears to be the strongest predictor of long-term mortality.18,19
Residual aortic regurgitation has been associated with a worse prognosis after TAVI. In a single-center study of 400 patients, mortality was higher in patients with grade 3 or 4 aortic regurgitation than in those with grade 2. In addition, these patients had higher mortality than those with grade 0 or 1 residual aortic regurgitation.20 In another registry of 167 patients, the residual aortic regurgitation rate was 67%, which was at least moderate in 14.4%. This degree of residual aortic regurgitation was associated with mortality during follow-up. Moreover, a gradient of =18mmHg between the aortic diastolic and left ventricular end-diastolic blood pressures was a predictor of mortality with an area under the receiver operating characteristic curve of 0.97.21 Similarly, another study examined the aortic regurgitation index, defined as the quotient of the difference between the aortic diastolic and left ventricular end-diastolic blood pressures over the systolic blood pressure×100. An index<25 was an independent predictor of 1-year mortality.22 The association between paravalvular regurgitation and mortality is maintained at the 5-year follow-up point.23 Finally, in the PARTNER study, even a mild perivalvular leak was associated with an increase in the 2-year follow-up mortality rate.24 In our registry, rates of postdilatation and at least moderate aortic regurgitation were higher with CoreValves than with Edwards valves, which is in agreement with data from other registries.12,13 Nonetheless, no association was found between valve type and mortality.
Given that we did not have all the data on preprocedural mitral regurgitation, we were unable to include it in the multivariate analysis. However, we found a noteworthy association between preprocedural mitral regurgitation and in-hospital and mid-term mortalities in the univariate analysis. This finding is in line with that of the European Registry, which included 4571 patients from 10 countries and which found mitral regurgitation=2, implant failure, age, and EuroSCORE to be predictors of in-hospital mortality.25 Mitral regurgitation is common in patients with aortic stenosis and increases the risk of aortic replacement, both surgical and transcatheter. In the PARTNER B study,1 up to 22% of the patients showed concomitant moderate or severe mitral regurgitation, which was associated with greater early mortality; however, the benefit was greater with TAVI than with standard therapy. In addition, in the PARTNER A study, 20% of the patients showed moderate-severe mitral regurgitation, which was also associated with greater procedure-related mortality; however, mortality was lower in the TAVI group than in the surgical group (24.2% vs 35%, respectively).2 Although most studies have reported an improvement in mitral regurgitation after aortic stenosis treatment, this benefit may be restricted to functional mitral regurgitation.26 The severity of mitral regurgitation primarily depends on the regurgitant orifice involved and the systolic pressure gradient between the left ventricle and the left atrium.27 Afterload reduction following aortic valve replacement can cause positive ventricular remodeling, with a reduction in left ventricular volume that can help coaptation of the mitral leaflets.28 Accordingly, mitral regurgitation improvement is less probable in patients with structural mitral valve disease with deformed leaflets and significant annulus calcification.29
LimitationsNational registry participation is voluntary and is thus subject to inclusion bias. However, this registry can be considered to be representative of the current situation in Spain, because it has obtained data on 80% of the valves implanted during the inclusion period. In addition, data on the success and complications rates are similar to those of other national European registries.11–14,25
We were unable to collect clinically relevant variables (such as mitral regurgitation or chronic obstructive pulmonary disease) from all patients. Accordingly, these variables were omitted from the final multivariate analysis.
The inclusion rate and follow-up duration of patients implanted with valves via the transapical approach were significantly lower than those treated by the transfemoral approach, which could indicate an inclusion bias for these types of valves and approaches. Similarly, a few centers did not include all valves implanted during the registration period, which could also have cause some type of inclusion bias.
The events were reported by the investigators dbut were not independently reviewed. In addition, there is currently no way to perform independent audits of a certain percentage of the patients and consequently we cannot rule out the possibility of a certain degree of under- or over-registration of events. Nonetheless, this study involves the first national registry with individualized data of patients treated with this recent technique.
Finally, the different baseline characteristics could have influenced the clinical course of the patients who were treated with different valves or approaches (Figs. 1A and B). To minimize this potential bias, we included the types of valves and approaches in the multivariate analysis that did not appear in the model as predictors of events.
CONCLUSIONSThe TAVI national registry is a multicenter registry of individual patients that obtained data on 80% of transcatheter aortic valves implanted in Spain between 2010 and 2011. This registry has described for the first time the predictors of in-hospital and mid-term mortality after implantation. The appearance of complications during the procedure, reflected by the rate of conversion to surgery, comorbidities, and significant mitral regurgitation, was associated with in-hospital mortality, whereas residual aortic regurgitation was, along with the above factors, a factor associated with mortality during follow-up. The prognosis of these high-risk patients could be improved by considering comorbidities in patient selection and by minimizing the degree of residual aortic regurgitation to optimize the results of the procedure.
CONFLICTS OF INTERESTNone declared.
We thank Drs. Matías Feldman, María José Pérez-Vizcayno, and Luis Alonso Pulpón for their dedication to the TAVI Committee and their assistance with the drafting of this manuscript.
Manel Sabaté was a member of the Board of the Working Group on Cardiac Catheterization and Interventional Cardiology of the Spanish Society of Cardiology from 2009 to 2012.
Sergio Cánovas, Juan José Goiti, and Alberto Igual are members of the Spanish Society of Thoracic-Cardiovascular Surgery.
José María Hernández, Eulogio García, and Rosana Hernández-Antolín are members of the Working Group on Cardiac Catheterization and Interventional Cardiology of the Spanish Society of Cardiology.
Luis Alonso Pulpón is a clinical cardiologist at the Hospital Puerta de Hierro, Majadahonda, Madrid.
Matías Feldman was project manager of the TAVI Committee in 2010-2011.
María José Pérez-Vizcayno is project manager of the TAVI Committee in 2011-2013.
Garikotz Lasa Larraya and Miren Telleria Arrieta (Clínica Guipúzcoa de Donosti, San Sebastián). Gonzalo Pradas and Andrés Íñiguez (Complejo Hospitalario Universitario de Vigo, Vigo). Nicolás Vázquez González and José Joaquín Cuenca Castillo (Complejo Hospitalario Universitario A Coruña, A Coruña). Ramiro Trillo Nouche and Diego López Otero (Complejo Hospitalario de Santiago de Compostela, Santiago de Compostela). Javier Zueco and Dae-Hyun Lee (Hospital Universitario Marqués de Valdecilla, Santander). Federico Gimeno and Enrique Fulquet (Hospital Clínico Universitario de Valladolid, Valladolid). Vicenç Serra García and Bruno García del Blanco (Hospital Universitari Vall d’Hebron, Barcelona). Raquel del Valle Fernández and Pablo Avanzas Fernández (Hospital Universitario Central de Asturias, Oviedo). Raúl Moreno and Ulises Ramírez (Hospital Universitario La Paz, Madrid). Mariano Valdés Chávarri and Eduardo Pinar Bermúdez (Hospital Virgen de la Arrixaca, Murcia). Alberto Berenguer Jofresa (Hospital General, Valencia). Rafael Ruiz-Salmerón and Luis Felipe Valenzuela García (Hospital Virgen de La Macarena, Sevilla). Eduardo Molina Navarro and Norberto Herrera Gómez (Hospital Virgen de las Nieves, Granada). Cristóbal A. Urbano Carrillo and José L. Castillo Castro (Hospital Regional Universitario Carlos Haya, Málaga). Ángel Cequier Fillat and Rafael Romaguera (Hospital Universitari de Bellvitge, L’Hospitalet de Llobregat). Ignacio Cruz González and Javier Martín Moreiras (Complejo Hospitalario de Salamanca, Salamanca). Antonio Merchán and José Ramón González (Hospital Universitario Infanta Cristina, Badajoz). Agustín Albarrán and Jorge Centeno (Hospital 12 de Octubre, Madrid). Manuel Castellá (Hospital Clínic, Barcelona). Patricio Llamas and Vicente Mainar (Hospital General, Alicante). Roberto Blanco Mata and Juan Alcíbar Villa (Hospital de Cruces, Baracaldo). José Miguel Borrego and Ángel Sánchez González (Hospital Virgen del Rocío, Sevilla). Felipe Fernández-Vázquez and Mario Castaño Ruiz (Complejo Asistencial Universitario [CAULE], León). José María Aguirre Salcedo and Joseba Zuazo Meabe (Hospital de Basurto, Basurto). José Moreu Burgos and Fernando Pajín Valbuena (Hospital Virgen de la Salud, Toledo). Luis Felipe Navarro del Amo and Antonio Piñero Lozano (Fundación Jiménez Díaz, Madrid). Juan Francisco Oteo Domínguez and Javier Goicolea Ruigómez (Hospital Puerta de Hierro-Majadahonda, Madrid). José F. Díaz Fernández and Rosa Cardenal Piris (Hospital Juan Ramón Jiménez, Huelva). Luisa Salido and José Garrido (Hospital Ramón y Cajal, Madrid). Antoni Serra Peñaranda and Jose María Padró (Hospital de la Santa Creu i Sant Pau, Barcelona). Francisco Ten and Juan Margarit (Hospital La Fe, Valencia). Eduard Fernández Nofrerías and Xavier Carrillo Suárez (Hospital Universitari Germans Trias i Pujol, Badalona). Román Lezaun and Raúl Ramallal (Hospital de Navarra, Pamplona). Bernhard Seidelberger and Amparo Benedicto Buendía (Hospital de la Princesa, Madrid). Enrique Rodríguez-Hernández, Javier Cobiella, and Pilar Jiménez-Quevedo (Hospital Universitario Clínico San Carlos, Madrid). Miguel Duch (Hospital Clínico Virgen de la Victoria, Málaga). Jaime Elízaga Corrales, Francisco Fernández-Avilés Díaz, and Ángel González-Pinto (Hospital Gregorio Marañón, Madrid). Ignacio Gallo, Juan José Goiti, Mariano Larman, and Alberto Sáenz (Clínica Guipúzcoa de Donosti, San Sebastián). José Antonio Baz, Darío Duran, Víctor Jiménez, and Javier Montoto (Complejo Hospitalario Universitario de Vigo, Vigo). Jorge Salgado (Complejo Hospitalario Universitario A Coruña, A Coruña). Ángel Luis Fernández (Complejo Hospitalario de Santiago de Compostela, Santiago de Compostela). Iván García and José María de la Torre-Hernández (Hospital Universitario Marqués de Valdecilla, Santander). Alberto San Román (Hospital Clínico Universitario de Valladolid, Valladolid). Alberto Igual (Hospital Universitari Vall d’Hebron, Barcelona). César Morís (Hospital Universitario Central de Asturias, Oviedo). Nieves Montoro and José María Mesa (Hospital Universitario La Paz, Madrid). Ramón Arcas (Hospital Virgen de la Arrixaca, Murcia). Óscar Gil Albarova, Juan Vicente Vilar Herrero, Darío Sanmiguel Cervera, Salvador Morell Cabedo, and Juan Martínez León (Hospital General, Valencia). José Miguel Barquero (Hospital Virgen Macarena, Sevilla). Rafael Melgares Moreno (Hospital Virgen de las Nieves, Granada). Joan Antoni Gómez Hospital and Miguel Ángel Sánchez Corral (Hospital Universitari de Bellvitge, L’Hospitalet de Llobregat). Fermín González and José Ramón López-Mínguez (Hospital Universitario Infanta Cristina, Badajoz). Juan Tascón and José María Cortina (Hospital 12 de Octubre, Madrid). Rut Andrea, Victoria Martín-Yuste, Bárbara Vidal, Joaquim Cevallos, Guillermina Fita, and Matías Feldman (Hospital Clínic, Barcelona). Pascual Bordes and Juan Meseguer Oller (Hospital General, Alicante). José Ignacio Aramendi (Hospital de Cruces, Baracaldo). José María Cubero (Hospital Virgen del Rocío, Sevilla). Alejandro Diego Nieto and Javier Gualis (Complejo Asistencial Universitario [CAULE], León). Roberto Sáez Moreno, Leire Andraka Ikazuriaga, Vanessa Estévez Flórez, and Abel Andrés Morist (Hospital de Basurto, Basurto). Alfonso Cañas (Hospital Virgen de la Salud, Toledo). Antonio Gómez Menchero (Hospital Juan Ramón Jiménez, Huelva). Antonio Epeldegui, Ignacio García Andrade, and Jaime Pey (Hospital Ramón y Cajal, Madrid). Marcelo Jiménez (Hospital de la Santa Creu i Sant Pau, Barcelona). José Anastasio Romero and Elena Sánchez (Hospital La Fe, Valencia). Josepa Mauri, Carolina Oliete, and Xavier Ruyra (Hospital Universitari Germans Trias i Pujol, Badalona).
In accordance with the wishes of the authors and the editors, this article will be published simultaneously and in its entirety in the journal Cirugía Cardiovascular (http://dx.doi.org/10.1016/j.circv.2013.10.003).
The members of the 2010-2013 TAVI Committee and the collaborators of the TAVI National Group are listed in the Appendices.