Acute pulmonary embolism (PE) is the third leading cause of cardiovascular mortality. In Spain, it has an annual incidence of 154 cases per 100 000 inhabitants.1 The mortality rate for massive PE (cardiogenic shock, systolic blood pressure <90mmHg, and organ hypoperfusion) can be as high as 60%. Catheter-directed therapy is an alternative to systemic fibrinolysis in refractory cases or patients with a high risk of bleeding.
A recent study of catheter-directed therapy in patients with high-risk PE reported a success rate of 80% and a complication rate of less than 5%.2 Extracorporeal cardiopulmonary resuscitation (CPR) consists of the rapid deployment of venoarterial extracorporeal membrane oxygenation (VA-ECMO) to provide circulatory support when conventional CPR fails to achieve sustained return of spontaneous circulation. Clinical practice guidelines recommend considering VA-ECMO as rescue therapy for selected patients. In patients with massive PE who develop refractory cardiogenic shock or experience cardiorespiratory arrest, VA-ECMO can be used to maintain appropriate hemodynamics and organ perfusion, serving as a bridge to stabilization or reperfusion.
We describe our initial experience with percutaneous mechanical thrombectomy and VA-ECMO. Cannulation was performed by a specialist cardiovascular technologist and an ECMO-experienced intensivist in the catheterization laboratory using arterial return cannulas (17-19 Fr) and venous drainage cannulas (21-25 Fr) (Bio-Medicus NextGen; Medtronic, United States). The Novalung ECMO circuit (Fresenius, United States) was primed. Vascular access was achieved percutaneously under ultrasound guidance with fluoroscopic confirmation using a unilateral or bilateral femorofemoral approach. In all cases, a 6-Fr distal perfusion cannula was placed in the superficial femoral artery. After initiation of VA-ECMO (flow rate of 3-4 L/min), thrombectomy was performed via the femoral vein contralateral to the venous drainage cannula. A 24-Fr Gore DrySeal Flex introducer sheath (Gore Medical, United States) and the FlowTriever 24 Curve system (Inari Medical, United States) were used to maximize aspiration. ECMO support was interrupted momentarily during the removal of the 24-Fr introducer sheath to prevent air from entering the system. Although the ECMO system is designed to eliminate this risk, a large volume of air entering the system could disrupt the flow of blood through the centrifugal pump, compromising life support. Vascular closure was achieved using compression and figure-of-8 sutures. The patients were transferred to the coronary care unit on completion of the thrombectomy.
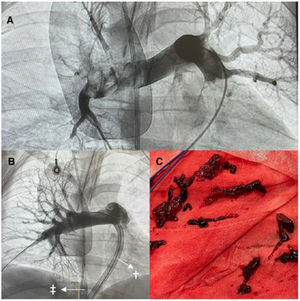
In 2023, we performed 4 mechanical thrombectomies with VA-ECMO: 2 in patients with cardiorespiratory arrest and 2 in patients with refractory cardiogenic shock in whom fibrinolysis had been ineffective. The cases were diagnosed by transthoracic echocardiography and confirmed by angiography after initiation of ECMO. Support was started within 4hours of diagnosis in all cases. All patients received norepinephrine at a dosage of >1μg/kg/min. The mean patient age was 51 years (table 1). The median time on ECMO was 44hours. Perfusion was restored in the main pulmonary arteries after the procedure as thrombectomy was successful in all cases (figure 1A,B). The ECMO blood flow rate was not reduced during aspiration. Apart from the patients’ dependence on the life support system, the venous cannula was positioned at the entrance of the vena cava (figure 1B, arrow, double dagger) and the aspiration device in the pulmonary arteries (figure 1B, arrow, dagger). The decision not to adjust the flow rate did not affect the success of the procedure (figure 1B,C) or cause air to enter the ECMO circuit. Three of the 4 patients were successfully weaned off VA-ECMO. The fourth patient died during support. She experienced flow problems, probably due to a loss of pulsatility, and developed intestinal ischemia. Two of the patients had a favorable neurological outcome (Cerebral Performance Category 1 to 2). The in-hospital survival rate was 50%. The most common complication was access site bleeding. All the patients required blood transfusion and 1 of them experienced major bleeding. Decannulation was performed by the vascular surgery team.
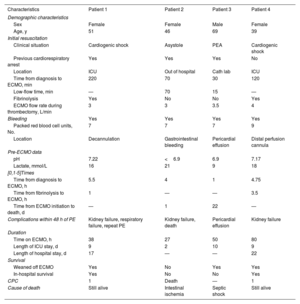
Patient characteristics and clinical outcomes
| Characteristics | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Sex | Female | Female | Male | Female |
| Age, y | 51 | 46 | 69 | 39 |
| Initial resuscitation | ||||
| Clinical situation | Cardiogenic shock | Asystole | PEA | Cardiogenic shock |
| Previous cardiorespiratory arrest | Yes | Yes | Yes | No |
| Location | ICU | Out of hospital | Cath lab | ICU |
| Time from diagnosis to ECMO, min | 220 | 70 | 30 | 120 |
| Low-flow time, min | — | 70 | 15 | — |
| Fibrinolysis | Yes | No | No | Yes |
| ECMO flow rate during thrombectomy, L/min | 3 | 3 | 3.5 | 4 |
| Bleeding | Yes | Yes | Yes | Yes |
| Packed red blood cell units, No. | 7 | 7 | 7 | 9 |
| Location | Decannulation | Gastrointestinal bleeding | Pericardial effusion | Distal perfusion cannula |
| Pre-ECMO data | ||||
| pH | 7.22 | <6.9 | 6.9 | 7.17 |
| Lactate, mmol/L | 16 | 21 | 9 | 18 |
| [0,1-5]Times | ||||
| Time from diagnosis to ECMO, h | 5.5 | 4 | 1 | 4.75 |
| Time from fibrinolysis to ECMO, h | 1 | — | — | 3.5 |
| Time from ECMO initiation to death, d | — | 1 | 22 | — |
| Complications within 48 h of PE | Kidney failure, respiratory failure, repeat PE | Kidney failure, death | Pericardial effusion | Kidney failure |
| Duration | ||||
| Time on ECMO, h | 38 | 27 | 50 | 80 |
| Length of ICU stay, d | 9 | 2 | 10 | 9 |
| Length of hospital stay, d | 17 | — | — | 22 |
| Survival | ||||
| Weaned off ECMO | Yes | No | Yes | Yes |
| In-hospital survival | Yes | No | No | Yes |
| CPC | 1 | Death | — | 1 |
| Cause of death | Still alive | Intestinal ischemia | Septic shock | Still alive |
Cath lab, catheterization laboratory; CPC, Cerebral Performance Category; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; PE, pulmonary embolism; PEA, pulseless electrical activity.
In a recent review of data from the Extracorporeal Life Support Organization, extracorporeal CPR was found to be an independent predictor of in-hospital mortality (OR=3.67; 95% CI, 1.46-9.2) in a group of 821 patients with PE who underwent ECMO (88% with VA support and 28% with extracorporeal CPR).3 The use of reperfusion therapies has been linked to a decrease in ECMO duration and an increased likelihood of successful weaning. The American Heart Association recommends early initiation of VA-ECMO in patients with massive PE and refractory shock when fibrinolysis is ineffective, as these patients have a high risk of cardiorespiratory arrest before or during thrombectomy.4 All 4 patients in the present series developed severe biventricular dysfunction and experienced intermittent loss of pulsatility during thrombectomy. VA-ECMO was therefore essential for preventing a likely irreversible cardiorespiratory arrest.5 Hobohm et al.6 recently reported that embolectomy and VA-ECMO were used only in a minority of patients but were associated with a lower likelihood of in-hospital mortality (OR=0.5; 95% CI, 0.41-0.61).
In conclusion, early VA-ECMO support in patients with massive PE who develop refractory cardiogenic shock or experience cardiorespiratory arrest is a feasible and effective strategy for maintaining adequate hemodynamics, and within this setting, mechanical thromboaspiration is considered safe and effective.
FUNDINGNone
ETHICAL CONSIDERATIONSThis study was conducted in accordance with international clinical research recommendations (World Medical Association Declaration of Helsinki). Informed consent was not deemed necessary as the procedures were emergency interventions. Verbal consent, however, was obtained from the patients’ relatives. All possible sex and gender biases were taken into account when writing this article.
USE OF ARTIFICIAL INTELLIGENCEArtificial intelligence was not used for this article.
AUTHORS’ CONTRIBUTIONSAll the authors had access to the data and contributed to preparing this manuscript. R. García del Moral drafted the article and J. Caballero-Borrego revised it. All the authors contributed to the conceptualization of the study, data curation, formal analysis, research, methodology, validation, and revision.
CONFLICTS OF INTERESTNone.