Transfemoral transcatheter aortic valve implantation (TAVI) is an established therapy for patients with severe inoperable aortic stenosis or high surgical risk.
Since the first TAVI implantation in humans in 2002,1 various models of this prosthesis have been developed, with progressive technical improvements and a reduction in the profile of the delivery system. One of the latest generations of this type of valve is the Edwards-SAPIEN 3 (Edwards Lifesciences; Irvine, California, United States), a prosthesis designed for easy placement within the valve plane and an improved outcome of the intervention.
This valve system has been designed with 14-F (for 23 and 26mm valves) and 16-F (29mm valves) delivery systems, due to the simplified structure of the chrome-cobalt mesh, the reduced thickness of the struts and the improved folding system, while maintaining the radial strength and circularity of the stent. It also incorporates an inner and outer polyethylene terephthalate skirt designed to minimize residual perivalvular leaks. The delivery system has a double joint, which improves control over the positioning of the valve with a central radiopaque marker in the ball; this marker facilitates alignment within the valve plane.
In this article, we describe our initial experience of transfemoral TAVI using the Edwards-SAPIEN 3 prosthesis, analyzing the first 5 interventions carried out in Spain by the same operator in 2 centers.
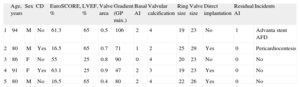
Clinical characteristics and procedures are described in the Table. In all patients, a guidewire was advanced from the contralateral femoral at the beginning of the procedure, according to a previously described technique.2 The Prostar XL (Abbott Vascular, Santa Clara, California, United States) percutaneous closure system was used. Valvuloplasty was performed in 2 patients (at the manufacturer's request in 1, because it was the first time this procedure had been performed in Spain) and direct implantation in the remaining 3, following a previously described protocol.3–5 The procedure was successfully completed in all patients without significant residual periprosthetic aortic insufficiency, and with a good immediate hemodynamic response. In 1 patient, a coated Advanta (Grifolds) stent was implanted due to incomplete closure of the arteriotomy in the right common femoral artery. In 4 of these 5 patients (1, 2, 3, and 4), we would have had great difficulty in guaranteeing the success of the intervention using the previous prosthesis model, given the vascular access. In another patient, pericardiocentesis was performed due to perforation by the pacemaker lead. In a final patient, severe damage to the proximal circumflex (not detected in the initial coronary angiography) was identified by post-implantation aortographic inspection, and we decided to perform immediate angioplasty with stenting, catheterizing the common trunk via the valve structure.
Description of Patients and Procedure for Transfemoral Implantation of the Edwards-SAPIEN 3 Aortic Prosthesis
| Age, years | Sex | CD | EuroSCORE, % | LVEF, % | Valve area | Gradient (GP máx.) | Basal AI | Valvular calcification | Ring size | Valve size | Direct implantation | Residual AI | Incidents | |
| 1 | 94 | M | No | 61.3 | 65 | 0.5 | 106 | 2 | 4 | 19 | 23 | No | 1 | Advanta stent AFD |
| 2 | 80 | M | Yes | 16.5 | 65 | 0.7 | 71 | 1 | 2 | 25 | 29 | Yes | 0 | Pericardiocentesis |
| 3 | 86 | F | No | 55 | 25 | 0.8 | 90 | 0 | 4 | 20 | 23 | No | 0 | No |
| 4 | 91 | F | Yes | 63.1 | 25 | 0.9 | 47 | 2 | 3 | 19 | 23 | Yes | 0 | No |
| 5 | 80 | M | No | 16.5 | 65 | 0.4 | 80 | 2 | 4 | 22 | 26 | Yes | 0 | No |
CD, coronary disease; LVEF, left ventricular ejection fraction; AI, aortic insufficiency; F, female; M, male.
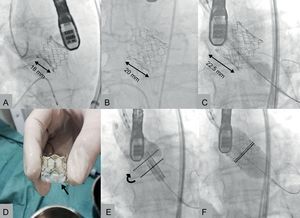
The mean hospital stay was 5.4 (SD, 3.6) (range, 3-12) days. No patient required pacemaker implantation following the procedure. Thirty-day mortality was zero and all patients remained in functional class I. Figures A-C show angiographic images of the various sizes of prostheses implanted.
Edwards-SAPIEN 3 prostheses implanted, 23 (A), 26 (B) and 29mm (C). Placement of the valve at the level of the ring (E) with elevation of the valve after inflation of the balloon (F). The solid line indicates the central zone of the prosthesis, marked by the radiopaque line of the balloon. The dotted line indicates the ideal final position that this marker should reach. Image of a 23mm prosthesis before implantation, with polyethylene terephthalate outer skirt (D, arrow).
Implantation of the Edwards SAPIEN 3 is simpler than with previous Edwards models, although we note some practical considerations. The valve should be placed in a slightly deeper position in the outflow tract of the left ventricle than previous prostheses because the lower half is shortened during the final phase of inflation due to the design of the cells (Figure). Moreover, the structure of the prosthesis is longer, so it is advisable to make a proper assessment of the distance between the valve plane and the left coronary ostium.
This new prosthesis also has additional advantages over previous valves: the reduced diameter of the delivery system extends the range of patients who can undergo the transfemoral approach; moreover, the lower profile of the prosthesis favors direct implantation in most patients. Another of its most attractive features is the reduced residual periprosthetic aortic insufficiency, given the widely recognized relationship between this parameter and patient mortality during follow-up.6 Although some incidents occurred during the procedure and had to be resolved, it should be stressed that these were very high-risk patients with an average EuroSCORE I of >40 (42.5%), and who maintained an excellent clinical status after 30 days.
In conclusion, the SAPIEN 3 percutaneous aortic valve is an important advance in a new generation of valves that will broaden the spectrum of candidate patients for this therapeutic strategy, and is progressively establishing itself as a clear alternative to surgical valve replacement.