Interleukin (IL)-5 is an anti-inflammatory cytokine that has been demonstrated to be involved in cardiovascular diseases, including aortic aneurysm and heart failure. This study aimed to investigate the involvement of IL-5 in coronary artery disease (CAD) and the possible mechanisms.
MethodsWe analyzed IL-5 expression in human coronary artery specimens collected from CAD patients and deceased donors. Plasma IL-5, IL-17, and interferon-γ levels in CAD patients were detected using ELISA kits, with samples from chest pain patients (non-CAD) as controls. Mouse CD4+T helper (Th) cells were separated, and the effect of IL-5 on Th1, regulatory T cell and Th17 differentiation and mRNA levels of their characteristic cytokines were detected using flow cytometry and reverse transcription-quantitative polymerase chain reaction, respectively.
ResultsIL-5 was significantly decreased in the coronary plaque of CAD patients compared with the deceased donors group, and IL-5 was mainly derived from macrophages in the coronary artery plaque. Compared with the non-CAD group, plasma IL-5 levels in the CAD groups were significantly lower, and the sequence from high to low was stable angina pectoris, unstable angina pectoris, and acute myocardial infarction. Binary linear regression analysis showed that IL-5 was independently correlated with the occurrence of CAD. Recombinant mouse IL-5 treatment decreased Th1 and Th17 levels and mRNA expression of their characteristic cytokines in oxidized low-density lipoprotein-treated CD4+Th cells.
ConclusionIL-5 levels were decreased in CAD patients and inhibited oxidized low-density lipoprotein Th1 and Th17 differentiation in vitro.
Keywords
Coronary artery disease (CAD) is a common but severe cardiovascular disease and is the leading cause of morbidity and mortality worldwide. CAD is the further development of atherosclerosis, and its main pathological process is the activation of inflammatory reactions and the coagulation system.1 A variety of inflammatory cells and cytokines contribute to the thinning of the fibrous cap and enlargement of the lipid core, thus promoting the formation and rupture of vulnerable plaques.2
Interleukins (IL) are inflammatory-related cytokines that play critical roles in the formation of atherosclerosis/CAD. IL-1 exerts inflammatory effects on macrophages, endothelial cells and vascular smooth muscle cells, whereas gene deletion of IL-1β or IL-1R decreases atherosclerotic lesion development.3 Additionally, IL-6 is independently associated with the prevalence and severity of coronary atherosclerosis.4 Moreover, IL-18 can increase lesion development through the enhancement of an inflammatory response involving interferon (IFN)-γ-dependent mechanism,5 whereas IL-10 is defined as a protective factor in both atherosclerotic lesion formation and stabilization.6
IL-5 is an anti-inflammatory cytokine mainly secreted by inflammatory cells, including T lymphocytes, mast cells, macrophages, and eosinophils.7,8 IL-5 is widely known as a major factor in eosinophil growth, maturation and release from bone marrow. It has been noted that eosinophil counts were significantly increased in patients with vasospastic angina pectoris or CAD and the absolute number returned to normal after treatment.9,10 IL-5 could prolong eosinophil survival and plays critical roles in orchestrating and amplifying allergic response in asthma and hypereosinophilic syndrome.11 Moreover, Zhang et al.12 found that excessive IL-5 production could heighten the risk of subsequent severe respiratory infections in children with an atopic family history. Compared with immunocompetent mice, IL-5(-/-) mice showed less activation of eosinophils in the lungs aftr parasitic infection.13 Studies have demonstrated that IL-5 levels are elevated in patients with nonsmall cell lung cancer,14 and IL-5 also plays a role in the antiretroviral therapy of AIDS.15
IL-5 was also demonstrated to be involved in cardiovascular diseases. Liu et al.16 found that in allergic lung inflammation, plasma IL-5, IL-13 and transforming growth factor beta levels dropped, while inflammatory cell and eosinophil accumulation increased; these authors also found that IL-5 and transforming growth factor beta levels were reduced in mice with pre-established abdominal aortic aneurysms. IL-5 was also decreased in patients with chronic heart failure.17 Interestingly, it has been reported that asthmatic patients are at decreased risk of atherosclerosis, while plasma IL-5 is increased in patients with asthma.18
No data are available on IL-5 expression in CAD patients. Therefore, the aim of this study was to prospectively evaluate IL-5 expression in patients with CAD and explore possible mechanisms.
METHODSCollection of human coronary specimensCoronary artery tissue (n=10) was collected from the coronary arteries of patients with CAD who underwent heart transplant surgery. The control samples (n=7) were obtained from deceased donors who had had traffic accidents or strokes and were declared brain-dead by experienced clinicians. Donors had no history of cardiovascular disease, and the samples showed no signs of pathology. All the coronary tissues were collected by surgeons during heart transplant procedures at the People's Hospital of Guangxi Zhuang Autonomous Region (China). Written informed consent was obtained from families, and the study protocol was approved by the Medical Ethics Committee of the People's Hospital of Guangxi Zhuang Autonomous Region.
Western blotCoronary samples were lysed, and total protein was collected. After quantification using a bicinchoninic acid (BCA) Protein Assay Kit, 20μg protein were added to 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gel. The proteins were separated by electrophoresis and were then transferred to Immobilon-FL PVDF membranes. The membranes were blocked with 5% nonfat milk and incubated with anti-IL-5 antibody (Santa Cruz Biotechnology) and antiglyceraldehyde-3-phosphate dehydrogenase (GAPDH, Cell Signaling Technology) antibody at 4° C overnight, followed by incubation with secondary antibody at room temperature for 1 hour. The proteins were visualized and quantified using a 2-color infrared imaging system (Odyssey; LI-COR Biosciences). Protein expression levels were normalized to the GAPDH control.
Reverse transcription-quantitative polymerase chain reactionTotal mARN was collected after coronary tissue and cells were lysed using the TRIzol reagent. Then, complementary DNA (cDNA) was synthesized from 2μg of total mRNA using oligo (dT) primers and a reverse transcription kit according to the manufacturer's instructions. Polymerase chain reaction (PCR) amplifications were performed using a LightCycler 480 SYBR Green Master Mix (all from Roche). The primer sequences are listed in Table 1. The relative mRNA expression levels were measured and were normalized to GAPDH in the corresponding sample.
Reverse transcription-quantitative polymerase chain reaction primers used
| Gene | Forward primer(5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| IL-5 (human) | CCCACAAGTGCATTGGTGAA | CCTCAGAGTCTCATTGGCTATCAG |
| IL-17 (human) | CTCTGTGATCTGGGAGGCAAA | CTCTTGCTGGATGGGGACA |
| IFN-γ (human) | GCAGGTCATTCAGATGTAGCGG | TGTCTTCCTTGATGGTCTCCACAC |
| GAPDH (human) | TTGTCAAGCTCATTTCCTGGT | TTACTCCTTGGAGGCCATGTA |
| IL-17 (mouse) | TCCAGAAGGCCCTCAGACTA | AGCATCTTCTCGACCCTGAA |
| IFN-γ (mouse) | ACTGGCAAAAGGATGGTGAC | TGAGCTCATTGAATGCTTGG |
| TBX21 (mouse) | ATTGGTTGGAGAGGAAGCGG | TGTGCACCCTTCAAACCCTT |
| IL-18 (mouse) | ATGCTTTCTGGACTCCTGCC | GTCTGGTCTGGGGTTCACTG |
| RORγT (mouse) | CTGTCCTGGGCTACCCTACT | CCACTTGTTCCTGTTGCTGC |
| IL-23 (mouse) | AATAATGTGCCCCGTATCCA | CATGGGGCTATCAGGGAGTA |
| GAPDH (mouse) | AACTTTGGCATTGTGGAAGG | CACATTGGGGGTAGGAACAC |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IFN-γ, interferon-γ; IL, interleukin; RORy, retinoic acid receptor-related orphan receptor gamma; TBX21, T-box transcription factor.
All the coronary samples were fixed with 4% neutral paraformaldehyde, and after being embedded in paraffin, the samples were cut into 5 μm slices and mounted onto slides. Immunofluorescence staining was used to detect IL-5 expression in each sample. To determine the source of IL-5, double immunofluorescence staining with the anti-CD4 antibody, anti-CD68 antibody, and anti-IL-5 antibody was performed.
Collection of human blood samplesWe selected a whole new set of consecutive patients with chest pain who were suspected to have CAD at the People's Hospital of Guangxi Zhuang Autonomous Region from June 2016 to December 2017. All patients underwent coronary angiography. According to the clinical symptoms and the results of the coronary arteriogram and electrocardiogram, the patients were divided into 4 groups: a) Non-CAD (NCAD, n=36) group. Inclusion criteria: chest pain was not accompanied by electrocardiogram changes, coronary stenosis, or coronary spasm when an intracoronary acetylcholine injection was given during coronary angiography; b) SAP group (n=51). Inclusion criteria: typical exertion-induced chest discomfort associated with down-sloping or a horizontal ST-segment depression> 1mm in an exercise test; c) UAP group (n=44). Inclusion criteria: chest pain at rest with definite ischemic electrocardiographic changes: ST-segment changes and/or T-wave inversions, and d) Acute myocardial infarction (AMI, n=29) group. Inclusion criteria: myocardial infarction confirmed by a significant rise in troponin I and creatine kinase MB levels.
The standard of grouping was based on a previous study by Lin et al.19 We excluded patients who had had an AMI within the previous 3 months, or unstable angina pectoris within the previous month.20 We also excluded patients with evidence of significant concomitant diseases, in particular, hemodynamic valvular heart disease, cardiomyopathy, known malignant diseases, and febrile conditions.21 The diagnosis was established the clinicians with extensive clinical experience. Gensini scores were used to assess stenosis in the coronary arteries. The detailed algorithm of Gensini scores has been previously described in by Ye et al.22
The patients themselves or their families provided written informed consent, and the study protocol was approved by the Medical Ethics Committee of the People's Hospital of Guangxi Zhuang Autonomous Region.
Measurement of plasma IL-5, IL-17, and IFN-γBlood samples were obtained upon patient arrival at the emergency unit. Blood samples were collected before coronary angiography was performed and before any treatment was provided. The time of onset for all patients was less than 6hours. The samples were centrifuged at 4000×g for 20minutes and then the supernatants were collected and stored at−80° C until the beginning of the experiments. The samples were removed from a−80° C environment and then thawed at room temperature. Plasma IL-5 (R&D Systems), IL-17, and IFN-γ (both from eBioscience) levels were determined using an enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's instructions.
Cell cultureMale adult C57BL/6 mice were anesthetized with 2% isoflurane, and the spleens were isolated. The splenic single cell suspension was prepared and cultured in RPMI 1640 (Gibco) complete culture medium. The Cluster of Differentiation 4 (CD4+) Th cells were positively selected using CD4 magnetic beads (Miltenyi Biotech) and an auto magnetic activated cell sorting separator.23 After activation by anti-CD3 and anti-CD28 (both from eBioscience), the CD4+Th cells were treated with oxidized low-density lipoprotein (50 μg/mL, Servicebio) or recombinant mouse IL-5 (rIL-5, Genetex). Then the cells were cultured in a humidified CO2 incubator with 5% CO2 at 37°C for 24hours.24,25
Th1 and Th17 measurementThe CD4+Th cells cultured above were collected and stained with FITC (fluorescein isothiocyanate) anti-CD4 (FITC-CD4). After treatment with fixation/permeabilization concentrate, the cells were stained with phycoerythrin (PE)-labeled anti-IFN-γ (PE-IFN-γ) and PE-labeled anti-IL-17 (PE-IL-17). The isotype controls were included for compensation and to confirm antibody specificity. Th1 cells were defined as CD4+/IFN-γ+, and Th17 cells were defined as CD4+/IL-17+. The cell stimulation cocktails and all of the flow cytometry antibodies were purchased from eBioscience and used according to the manufacturer's instructions.
Statistical analysisPlasma cytokine concentrations and clinical characteristics are expressed as medians (lower quartile to upper quartile) and were compared with the Mann-Whitney U test. The correlations between IL-17, IFN-γ, clinical characteristics and IL-5 were analyzed using the Spearman correlation analysis. Simple linear regression analysis and subsequent binary linear regression analysis were used to determine whether IL-5 was an independent biomarker of CAD. The data from cell culture are expressed as mean±standard deviation and were compared with Student t tests. All data were analyzed with SPSS 23.0 software, and P <.05 was considered statistically significant.
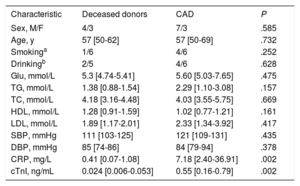
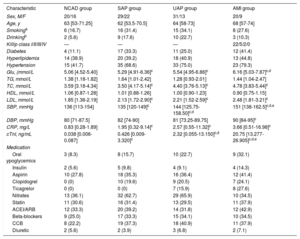
RESULTSClinical characteristics of patients providing coronary tissue samplesAmong the patients who provided coronary tissue, levels of cardiac troponin I (cTnI) and C-reactive protein (CRP) were significantly higher in the CAD groups than in the deceased donor group. No differences were found between the groups in other clinical characteristics, including age, sex, smoking, drinking, systolic blood pressure (SBP), fasting glucose (Glu), total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL), high-density lipoprotein (HDL), and diastolic blood pressure (DBP). Clinical data are listed in Table 2.
Clinical characteristics in coronary tissue sample donors
| Characteristic | Deceased donors | CAD | P |
|---|---|---|---|
| Sex, M/F | 4/3 | 7/3 | .585 |
| Age, y | 57 [50-62] | 57 [50-69] | .732 |
| Smokinga | 1/6 | 4/6 | .252 |
| Drinkingb | 2/5 | 4/6 | .628 |
| Glu, mmol/L | 5.3 [4.74-5.41] | 5.60 [5.03-7.65] | .475 |
| TG, mmol/L | 1.38 [0.88-1.54] | 2.29 [1.10-3.08] | .157 |
| TC, mmol/L | 4.18 [3.16-4.48] | 4.03 [3.55-5.75] | .669 |
| HDL, mmol/L | 1.28 [0.91-1.59] | 1.02 [0.77-1.21] | .161 |
| LDL, mmol/L | 1.89 [1.17-2.01] | 2.33 [1.34-3.92] | .417 |
| SBP, mmHg | 111 [103-125] | 121 [109-131] | .435 |
| DBP, mmHg | 85 [74-86] | 84 [79-94] | .378 |
| CRP, mg/L | 0.41 [0.07-1.08] | 7.18 [2.40-36.91] | .002 |
| cTnI, ng/mL | 0.024 [0.006-0.053] | 0.55 [0.16-0.79] | .002 |
CAD, coronary artery disease; CRP, C-reactive protein; cTnI, cardiac troponin I; DBP, diastolic blood pressure; F, female; Glu, fasting glucose; HDL, high-density lipoprotein; LDL, low-density lipoprotein; M, male; SBP, systolic blood pressure; TC, total cholesterol; TG, total triglycerides.
Data are expressed as proportions or median [interquartile range].
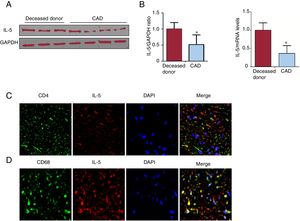
IL-5 was significantly lower in the coronary plaque of CAD patients than in that of deceased donors (Figure 1A-B). Both CD4+Th cells and macrophages were the source of IL-5, of which the macrophages were the main source (Figure 1C-D).
Expression and source of IL-5 in human coronary tissue. A The IL-5 levels in the coronary plaque of CAD patients and deceased donors were measured by western blotting. B. The IL-5 mRNA levels in these 2 groups were measured by RT-qPCR. C, D. The source of IL-5 was analyzed by double immunofluorescence staining in coronary tissue (400×). CAD, coronary artery disease; CD, cluster of differentiation; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; IL, interleukin; RT-qPCR, reverse transcription-quantitative polymerase chain reaction. * P < .05.
Levels of Glu, TC, SBP, LDL, CRP and cTnI were significantly lower in the NCAD group than in the other 3 groups. Other clinical characteristics, including age, sex, smoking and drinking, TG, HDL, and DBP, showed no obvious differences. None of the clinical characteristics was obviously different between SAP, UAP and AMI groups except SBP and cTnI. SBP and cTnI levels were highest in the AMI group, higher than in the UAP group, and were lowest in the SAP. Clinical data are listed in Table 3.
Clinical characteristics of patients providing blood samples
| Characteristic | NCAD group | SAP group | UAP group | AMI group |
|---|---|---|---|---|
| Sex, M/F | 20/16 | 29/22 | 31/13 | 20/9 |
| Age, y | 63 [53-71.25] | 62 [53.5-70.5] | 64 [58-73] | 68 [57-74] |
| Smokinga | 6 (16.7) | 16 (31.4) | 15 (34.1) | 8 (27.6) |
| Drinkingb | 2 (5.6) | 9 (17.6) | 10 (22.7) | 3 (10.3) |
| Killip class I/II/III/IV | — | — | — | 22/5/2/0 |
| Diabetes | 4 (11.1) | 17 (33.3) | 11 (25.0) | 12 (41.4) |
| Hyperlipidemia | 14 (38.9) | 20 (39.2) | 18 (40.9) | 13 (44.8) |
| Hypertension | 15 (41.7) | 35 (68.6) | 33 (75.0) | 23 (79.3) |
| Glu, (mmol/L | 5.06 [4.52-5.40] | 5.29 [4.91-6.36]c | 5.54 [4.95-6.86]c | 6.16 [5.03-7.87]c,d |
| TG, mmol/L | 1.38 [1.18-1.82] | 1.64 [1.01-2.42] | 1.28 [0.93-2.01] | 1.44 [1.04-2.47] |
| TC, mmol/L | 3.59 [3.18-4.34] | 3.50 [4.17-5.14]c | 4.40 [3.76-5.13]c | 4.78 [3.83-5.44]c |
| HDL, mmol/L | 1.06 [0.87-1.28] | 1.01 [0.88-1.26] | 1.00 [0.90-1.23] | 0.90 [0.75-1.15] |
| LDL, mmol/L | 1.85 [1.36-2.19] | 2.13 [1.72-2.90]c | 2.21 [1.52-2.59]c | 2.48 [1.81-3.21]c |
| SBP, mmHg | 136 [113-154] | 135 [120-149]c | 144 [125.75-158.50]c,d | 151 [138-162.5]c,d,e |
| DBP, mmHg | 80 [71-87.5] | 82 [74-90] | 81 [73.25-89.75] | 90 [84-95]c |
| CRP, mg/L | 0.83 [0.28-1.89] | 1.95 [0.32-9.14]c | 2.57 [0.55-11.32]c | 3.66 [0.51-16.98]c |
| cTnI, ng/mL | 0.038 [0.006-0.087] | 0.426 [0.009-3.320]c | 2.32 [0.055-13.150]c,d | 20.75 [13.277-26.905]c,d,e |
| Medication | ||||
| Oral ypoglycemics | 3 (8.3) | 8 (15.7) | 10 (22.7) | 9 (32.1) |
| Insulin | 2 (5.6) | 5 (9.8) | 4 (9.1) | 4 (14.3) |
| Aspirin | 10 (27.8) | 18 (35.3) | 16 (36.4) | 12 (41.4) |
| Clopidogrel | 0 (0) | 10 (19.6) | 9 (20.5) | 7 (24.1) |
| Ticagrelor | 0 (0) | 0 (0) | 7 (15.9) | 8 (27.6) |
| Nitrates | 13 (36.1) | 32 (62.7) | 29 (65.9) | 10 (34.5) |
| Statin | 11 (30.6) | 16 (31.4) | 13 (29.5) | 11 (37.9) |
| ACEI/ARB | 12 (33.3) | 20 (39.2) | 14 (31.8) | 12 (42.9) |
| Beta-blockers | 9 (25.0) | 17 (33.3) | 15 (34.1) | 10 (34.5) |
| CCB | 8 (22.2) | 19 (37.3) | 18 (40.9) | 11 (37.9) |
| Diuretic | 2 (5.6) | 2 (3.9) | 3 (6.8) | 2 (7.1) |
ACEI, angiotensin-converting enzyme inhibitor; AMI, acute myocardial infarction; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; CRP, C-reactive protein; cTnI, cardiac troponin I; DBP, diastolic blood pressure; Glu, fasting glucose; F, female; HDL, high-density lipoprotein; LDL, low-density lipoprotein; M, male; NCAD, non-coronary artery disease; SAP, stable angina pectoris; SBP, systolic blood pressure; TC, total cholesterol; TG, total triglycerides; UAP, unstable angina pectoris.
Data are expressed as proportions, No. (%), or median [interquartile range].
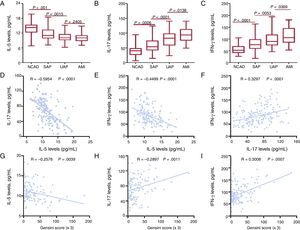
The results of ELISA showed that IL-5 levels were gradually decreased in the NCAD, SAP, and UAP groups, whereas no differences were found between the UAP and AMI groups (Figure 2A). Both IL-17 and IFN-γ levels gradually increased in the NCAD, SAP, UAP and AMI groups (Figure 2B-C). Spearman correlation analysis showed that IL-5 levels were negatively correlated with IL-17 levels and IFN-γ levels in CAD patients (Figure 2D-E). In addition, IL-17 levels were positively correlated with IFN-γ levels (Figure 2F).
Cytokines in CAD patients. Plasma IL-5 (A), IL-17 (B), and IFN-γ (C) concentrations in the NCAD and CAD groups. Correlations between IL-5 (D), IL-17 (E) and IFN-γ levels (F). Correlations between Gensini Score and plasma IL-5 (G), IL-17 (H) and IFN-γ (I) levels. AMI, acute myocardial infarction; CAD, coronary artery disease; IFN-γ, interferon-γ; IL, interleukin; NCAD, non-CAD; SAP, stable angina pectoris; UAP, unstable angina pectoris.
The correlation between IL-5, IL-17, IFN-γ levels and Gensini scores was detected to further determine whether the 3 cytokines are associated with the severity of CAD. The results showed that the Gensini score was positively correlated with IL-17 and IFN-γ levels but was negatively correlated with IL-5 (Figure 2G-I).
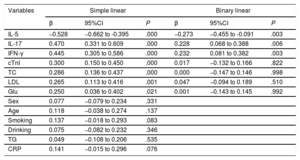
Simple and binary linear regression analysesThe results of the simple linear regression analysis showed that IL-5, IL-17, IFN-γ, cTnI, TC, LDL, and Glu exhibited a trend toward a correlation with the prevalence of CAD (P <.05). Then, the 3 cytokines and 4 clinical characteristics were analyzed by binary linear regression analysis, and the results showed that IL-5 (β=−0.273, 95%CI,−0.455 to−0.091; P=.003), IL-17 (β=0.228, 95%CI, 0.068 to 0.388; P=.006) and IFN-γ (β=0.232, 95%CI, 0.081 to 0.382; P=.003) were associated with the presence of CAD (as shown in Table 4).
Variables associated with the presence of coronary artery disease
| Variables | Simple linear | Binary linear | ||||
|---|---|---|---|---|---|---|
| β | 95%CI | P | β | 95%CI | P | |
| IL-5 | −0.528 | −0.662 to -0.395 | .000 | −0.273 | −0.455 to -0.091 | .003 |
| IL-17 | 0.470 | 0.331 to 0.609 | .000 | 0.228 | 0.068 to 0.388 | .006 |
| IFN-γ | 0.445 | 0.305 to 0.586 | .000 | 0.232 | 0.081 to 0.382 | .003 |
| cTnI | 0.300 | 0.150 to 0.450 | .000 | 0.017 | −0.132 to 0.166 | .822 |
| TC | 0.286 | 0.136 to 0.437 | .000 | 0.000 | −0.147 to 0.146 | .998 |
| LDL | 0.265 | 0.113 to 0.416 | .001 | 0.047 | −0.094 to 0.189 | .510 |
| Glu | 0.250 | 0.036 to 0.402 | .021 | 0.001 | −0.143 to 0.145 | .992 |
| Sex | 0.077 | −0.079 to 0.234 | .331 | |||
| Age | 0.118 | −0.038 to 0.274 | .137 | |||
| Smoking | 0.137 | −0.018 to 0.293 | .083 | |||
| Drinking | 0.075 | −0.082 to 0.232 | .346 | |||
| TG | 0.049 | −0.108 to 0.206 | .535 | |||
| CRP | 0.141 | −0.015 to 0.296 | .076 | |||
95%CI, 95% confidence interval; IL, interleukin; IFN-γ, interferon-γ; CRP, C-reactive protein; cTnI, cardiac troponin I; Glu, fasting glucose; LDL, low-density lipoprotein; TC, total cholesterol; TG, total triglycerides.
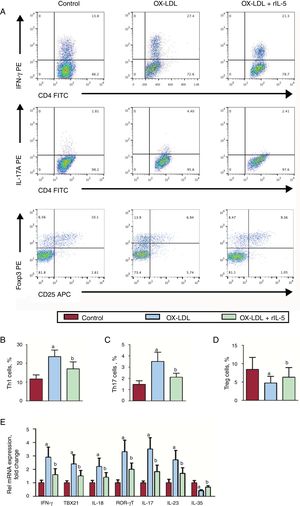
The results of flow cytometry analysis showed that oxidized low-density lipoprotein treatment significantly increased both Th1 and Th17 percentages, while regulatory T cell (Treg) numbers decreased (Figure 3A-D). These effects could be reversed by rIL-5. The mRNA of the characteristic Th1 and Th17 cytokines, such as IFN-γ, TBX21, IL-18, RORγT, IL-17, and IL-23, showed a similar trend as the Th1 and Th17 cells, respectively (Figure 3E).
Effect of IL-5 on Th1, Th17 and Treg differentiation in vitro. (A). Th1 and Th17 cells were identified among CD4+Th cells based on their expression of CD4+IFN-γ+ and CD4+IL-17+, respectively. The Th1 (B), Th17 (C) and Treg (D) levels in control, OX-LDL and OX-LDL+rIL-5 groups. (E). The IFN-γ, TBX21, IL-18, RORγT, IL-17, IL-23 and IL-35 mRNA levels in the 3 groups were measured by RT-qPCR. CD, cluster of differentiation; IFN-γ, interferon-γ; IL, interleukin; OX-LDL, oxidized low-density lipoprotein; PE, phycoerythrin; rIL-5, recombinant mouse IL-5; RoRγ, retinoic acid receptor-related orphan receptor gamma; RT-qPCR, reverse transcription-quantitative polymerase chain reaction; TBX, T-box transcription factor; Th, T helper cells; Treg, regulatory T cell. aP < .05 vs control group. bP < .05 vs OX-LDL group.
In the present study, we found for the first time that IL-5 was decreased in the coronary plaque of CAD patients and that macrophages were the main source of IL-5. In addition, plasma IL-5 levels were negatively correlated with the presence and severity of CAD, and rIL-5 treatment significantly inhibited Th1 and Th17 differentiation in vitro.
Previous studies found a negative correlation between IL-5 and the presence of cardiovascular diseases. Ishigami et al.7 reported that anti-IL-5 antibodies were positively associated with the development of atherosclerosis. IL-5 was also decreased in the patients with chronic heart failure, and decreased IL-5 levels were related to disease progression.17 In Kawasaki disease, IL-5 levels were negatively correlated with the occurrence of coronary artery lesions.26 However, IL-5 expression in CAD remains unknown; therefore, we measured IL-5 expression in the plasma and coronary plaques from CAD patients, and the results showed that IL-5 levels were significantly decreased. In addition, the results of the Gensini score showed that IL-5 levels were negatively associated with the extent of coronary stenosis.
Macrophages play a key role in the initiation and propagation of a variety of cardiovascular diseases, such as atherosclerosis,27 hypertensive heart disease,28 and heart failure.29,30 Macrophages are closely related to CAD; objective evidence is that abundant macrophages were seen from ruptured plaque.31 It has also been found that monocyte/macrophage-derived foam cells take up modified forms of LDL, thus contributing to atherogenesis.32 Th cells were associated with atherosclerosis, and IL-5 has also been reported to be derived from Th2 cells.7,33 Therefore, we further investigated the source of IL-5 in human coronary lesion tissue by performing double immunofluorescence staining with anti-CD68, anti-CD4, and anti-IL-5 antibodies. The results showed that both the macrophages and Th cells were the source of IL-5 in human coronary plaques and that IL-5 was mainly expressed in macrophages.
IL-17 and IFN-γ, the functional cytokines of Th1 and Th17, respectively, are the key factors in the chronic vascular inflammation typical of atherosclerosis.5,34,35 Macrophage-specific overexpression of IL-5 can also attenuate atherosclerosis induced by high fat food in LDL receptor-deficient mice,36,37 and an inverse association has been found between plasma IL-5 levels and carotid intima-media thickness.38 Considering the anti-inflammatory role of IL-5 and the critical role of Th1/ IFN-γ and Th17/IL-17 on the progression of atherosclerosis/CAD, we also measured plasma IL-17 and IFN-γ concentrations, and the results showed that both IL-17 and IFN-γ were increased in CAD patients and were positively correlated with the Gensini score. However, Spearman's correlation analysis showed that IL-5 levels were negatively associated with IL-17 and IFN-γ. Additionally, binary linear regression showed that IL-5 was negatively correlated with the presence of CAD. These data suggest that IL-5 may participate in CAD by regulating the secretion of inflammatory cytokines, such as IFN-γ and IL-17.
Rupture of atherosclerotic plaque is the pathological basis of most vascular complications, such as cerebral infarction and myocardial infarction. The stabilization of plaque is mostly determined by plaque composition.39 Stable plaques are mainly calcified and are more prone to rupture and fall off, while unstable plaques are mostly noncalcified. More and more inflammatory cells, such as CD3+T cells and CD68+macrophages, are recruited to the plaques and induce apoptosis of vascular smooth muscle cells, increasing plaque volume and the incidence of positive remodeling, finally resulting in the formation of vulnerable plaques.40,41 IL-5 has been proven to play atheroprotective roles in atherosclerosis, to promote the differentiation of B-1 cells to secrete more T15/EO6 antibody, which can block oxidized low-density lipoprotein uptake by macrophages and reduce the formation of foam cells.42 In this study, we found plasma IL-5 levels were lower in acute coronary syndrome patients than in patients with stable angina pectoris. This indicates that IL-5 was also related to the stabilization of coronary lesion plaques.
Previous studies showed that a changed ratio of Th1/Th2 lymphocytes is correlated with the development of atherosclerosis/CAD.43,44 Forkhead box P3+Tregs are the key players in maintaining immune homeostasis by responding to environmental cues to permit or suppress inflammation.45 In addition, both Th1/Treg and Th17/Treg levels are elevated in CAD patients and animal atherosclerosis models.45,46 These pieces of evidence demonstrated that Th1/Th2 and Th17/Treg imbalance plays a critical role in the development of atherosclerosis/CAD.
Considering the leading role of Th1, Th17 and Treg, and to further explore the mechanisms of IL-5 participating in atherosclerosis/CAD, we investigated the effect of IL-5 on Th1, Th17 and Treg differentiation in vitro. The results showed that rIL-5 treatment inhibited Th1 and Th17 differentiation, as well as the mRNA levels of their related cytokine. These data suggest that IL-5 plays a protective role in the atherosclerosis environment.
Study limitationsFirst, an animal experimental model of ischemic heart disease or infarction is needed to show the deleterious effect of IL-5KO or the beneficial effect of IL-5 stimulation in vivo. Second, our sample size is insufficient, and more patients are needed to validate our results. Third, the study participants enrolled from the hospital may have caused selection bias because inpatients are generally considered to have a more severe disease state. Finally, there was a lack of follow-up visits for CAD patients.
CONCLUSIONSThis study is the first to demonstrate a strong negative association between CAD and plasma IL-5 levels. The possible mechanism is that IL-5 attenuates atherosclerosis/CAD by inhibiting inflammation. In summary, we found that IL-5 is negatively associated with the presence of CAD, and inhibits Th1 and Th17 differentiation in vitro.
CONFLICTS OF INTERESTNone declared.
- –
Although there are many methods to help diagnose CAD, specific plasma markers are still needed for low-risk patients and convenience, especially for patients unable to undergo coronary angiography. The association between IL-5 levels and CAD remains unclear.
- –
In patients with an established diagnosis of CAD, we found that plasma IL-5 levels were decreased and IL-5 was negatively associated with proinflammatory cytokines.
- –
Recombinant IL-5 inhibited oxidized low-density lipoprotein-induced Th1 and Th17 differentiation in vitro.
- –
Inflammation is associated with the development of atherosclerosis/CAD, and Th1 and Th17 have been proven to be 2 important immune cells that promote the development of atherosclerosis/CAD. In our study, IL-5 inhibited oxidized low-density lipoprotein-induced Th1 and Th17 differentiation. This suggests that the occurrence of atherosclerosis/CAD may be related to an inability to effectively inhibit Th1 and Th17 differentiation caused by IL-5 deficiency, thus amplifying inflammation. IL-5 will definitely be helpful for the prevention and treatment of atherosclerosis/CAD in clinical practice.