Keywords
INTRODUCTION
Transcatheter closure of atrial septal defects (ASD) is recognized as a safe, efficient alternative to medical treatment and surgical intervention.1-4 Although surgery has been used for over 45 years and is considered the treatment of choice in patients with ostium secundum-type defects, nonsurgical ASD closure was first described as long ago as 1976.5 Although safe and effective, surgery entails thoracotomy-associated complications such as hemorrhage, arrhythmia, and physical and psychological after-effects in relatively young patients.6,7 Percutaneous occluding devices, previously of limited use for technical reasons, provide excellent alternative anatomic closure in adults today.8,9
In transcatheter ASD closure procedures, transesophageal echocardiography (TEE) is the standard imaging technique used to determine defect position and size, and guide intracardiac device implant procedures adequately.2-4,10 However, in adult patients TEE involves sedation or general anesthesia with orotracheal intubation, prolonging the procedure and increasing its complexity. The recent development of intracardiac echocardiography (ICE) increases the range of imaging techniques available to the cardiologist and constitutes a further option for transcatheter closure intervention guidance.11 Positioning the transductor in the right heart cavities offers excellent intracardiac anatomy definition. It has been used to guide transeptal needle puncture and in radiofrequency ablation procedures.12,13 Intracardiac echocardiography is reported as useful and safe when performed in transcatheter ASD closure procedures because it eliminates the need for TEE and general anesthesia, reduces intervention time, and gives the interventional cardiologist control of imaging without needing additional echocardiographic equipment.11,14-19
The present article describes our experience and the results of occluding device implantation in adult patients with ASD, guided solely by ICE to measure the defect, select device size and confirm correct device positioning.
METHODSPatients
From January 2002 to August 2006, 52 patients underwent transcatheter procedures for ASD closure (37 women and 15 men; age, mean [SD] [range], 39 [12] [22-74] years). Forty patients presented ostium secundum-type interatrial communication and 12 patent foramen ovale (PFO) with AS aneurysm. All patients had previously undergone TEE with a multiplane probe (Acuson Sequoia C-256, Siemens AG, Malvern, Pennsylvania, US) to confirm diagnosis, define the size, location, and number of ASD, and rule out other associated abnormalities (anomalous pulmonary vein drainage).
ICE
Intracardiac echocardiography was with a single-use, 9F-9MHz Ultra ICE catheter (EP Technologies, Boston Scientific Corp, Natick, Massachusetts, US) connected to a Clear View Ultra console (Boston Scientific Corp). This consists of a 9 F catheter with a 9 MHz rotating ultrasound element mounted at the tip. This obtains 9 MHz fixed frequency images, with a 4 cm deep radial field of vision. It is limited to obtaining single plane (horizontal) images and does not have Doppler.20,21
Under local anesthetic, the ICE catheter is introduced percutaneously using left femoral vein access with a 60 cm long, 55° precurved sheath (Convoy, Boston Scientific EP Technologies). The sheath tip is positioned in the right atrium (RA), and the transductor is manually maneuvered backwards and forwards with rotating movements. Standard planes are usually longitudinal, visualizing the 4 heart chambers—especially both atria and the AS—and transversal—at the level of the aortic valve plane.22 Maximum defect diameter (in the ASD) is measured in both planes. Imaging confirms that correct device deployment captures the ASD rims before final release (Figures 1 and 2). We did not need stretching balloons to decide on implant device size.
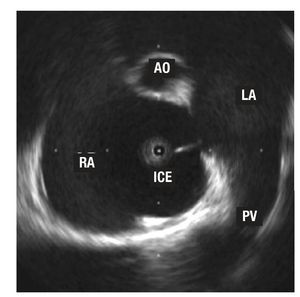
Figure 1. Image of an ostium secundum-type, interatrial wall defect. The tip of the intracardiac echocardiography (ICE) catheter is in the right atrium (RA). You can also see a right superior pulmonary vein (PV) and tangential cut of the aorta (AO) in the anterior plane.
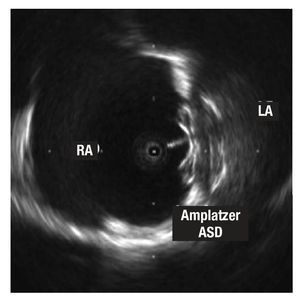
Figure 2. Image of the Amplatzer ASD device for atrial septal defects implanted in the septum. Note the larger size of the disc that remains in the left atrium (LA) and the echo of the cable connected to the disc in the right atrium (RA).
Devices
We used Amplatzer Septal Occluder (ASO) devices for ASD and Amplatzer Patent Foramen Ovale (PFO) Occluder devices for PFO (AGA Medical, Golden Valley, Minnesota, US). The self-centering device consists of a double disc of fine, Nitinol windings—one property of Nitinol is its thermal memory. The discs are connected by a 4 mm long waist, of 4-40 mm in diameter in the ASO; the left atrial disc is larger (14 mm more than the waist) than the right atrial disc (10 mm more than the waist). Both discs and waist contain polyester inserts that facilitate coagulation and defect occlusion. The device is screwed to the delivery wire transport cable and deployed thru a 6-12 F sheath positioned in the left atrium (LA) thru the ASD. A significant advantage of the device is that it can be recovered and repositioned as often as necessary prior to final release.
Closure and Follow-up Protocol
Under local anesthetic, we obtained bilateral femoral vein access, left for ICE and right to advance a 0.035", 260 cm guide thru the defect to the left superior pulmonary vein, aided, when necessary, by a 6 F multiuse catheter. After examining the ASD by ICE in at least 2 orthogonal projections, we chose an ASO 1-2 mm larger than the maximum diameter measured. In PFO, all devices were 25 mm (right atrial disc diameter). After positioning the sheath in the LA and withdrawing the guide, we advanced the device selected, deploying the distal disc in the LA and the proximal disc in the RA with radioscopic and ICE control. We confirmed correct disc positioning by ICE and performed the push-pull "Minnesota wiggle" maneuver (drawing the device towards both atria without releasing it)4 with a 30° left anterior oblique and 30° cranial tilt, before finally releasing the occluder.
All patients were receiving aspirin or clopidogrel; anticoagulation medication with intravenous sodium heparin at 100 U/kg was administered on initiating the procedure. We administered antibiotic prophylaxis with 1 g cephazolin on completing the procedure. We did not need TEE to visualize defects or decide on device size in any patient, so no one required general anesthesia or orotracheal intubation. Nor did we use the stretching balloon to decide what size device to implant, in any patient.
We conducted transthoracic echocardiography in the first 24 h to confirm correct device positioning and the absence of potential complications (thrombus, pericardial bleeding). All patients were discharged without immediate complications and with a drug regimen of 100 mg aspirin or 75 mg clopidogrel (for patients in sinus rhythm), both drugs combined in patients with PFO, or aspirin plus oral anticoagulation (for patients in atrial fibrillation). We detected no vascular complications in femoral access (all veinous except in 5 patients who also underwent diagnostic coronary angiography using femoral access during the procedure due to age and/or cardiovascular risk factors). At 6 months, patients underwent transthoracic echocardiography again; and at 6 and 12 months they attended clinical examination. This has since been repeated annually.
Statistical Analysis
Quantitative variables are expressed as mean (SD) and as percentages when appropriate.
RESULTS
Baseline and procedural data are summarized in Table. Devices were successfully implanted without immediate complications in 51 (98%) of the 52 patients. In 1 patient, ASD closure was not achieved as we were unable to correctly position the device despite several attempts with 2 different sized ASOs. The patient was referred for elective surgery which was performed without complications. Two patients had a cribriform septum: 1 with large ASD and 2 other defects in close proximity which were covered with a single 30 mm ASO; the other, with PFO and AS aneurysm, received 2 PFO devices in separate procedures.
In 7 patients with ASD, pulmonary systolic pressure prior to closure was 350 mm Hg (50-88 mm Hg). Left-to-right shunt had been documented by TEE and bidirectional shunt with systemic desaturation in some of them. Neither systemic hypotension nor other complications occurred after device deployment and in some patients saturation increased immediately.
In 4 patients, the anterosuperior AS rim was minimal (<3 mm) but we managed to anchor the device with a certain amount of retroaortic support, without protruding into or denting the aortic wall. In 1 patient, the inferior rim was practically nonexistent and the defect large but a 28 mm device was successfully implanted without the LA disc interfering with anterior mitral valve movement. In these patients, we performed TEE at 6-month follow-up to rule out malpositioning or aortic erosion.
Mean follow-up was 28 (9) (10-66) months. We recorded no significant vascular complications, pericardial bleeding, systemic embolic phenomena, or device embolization during follow-up. In 3 patients, symptomatic paroxysmal atrial fibrillation was recorded in the first month after implantation. In the 3 patients, prophylactic oral anticoagulation was begun and beta-blockers added; no further incidence was recorded. At 3 months, both treatments were ended.
At 6 months, transthoracic echocardiography confirmed correct implant positioning and color Doppler indicated the absence of significant residual shunting in all patients. Antiplatelet treatment was halted in patients with ASD unless another clinical indication suggested it should be continued. In patients with PFO, 1 of the 2 antiplatelet drugs was withdrawn. Clinical follow-up at 12 months was normal.
DISCUSSION
In the past, indication for ASD closure meant open-heart surgery was inevitable. Despite the benefits obtained, in adults, the serious complication rate is not inconsiderable (9%-13%).6,7,23,24 The technological evolution of percutaneous devices, experience of interventional cardiologists dedicated to these pathologies, and innovations in imaging techniques make percutaneous treatment of these defects efficient, safe and attractive.11,14-22
Although TEE defines the AS adequately, its use during interventional procedures in the cardiac catheterization laboratory implies the participation of other professionals (anesthetists for sedation or general anesthesia of the patient, echocardiographers to manage TEE) and prolongs intervention times.11,17 In our series, ICE use has successfully avoided the need for anesthesia and TEE in the catheterization laboratory, without diminishing the efficiency and safety of the procedure, and with the consequent comfort for patients.
The definition of intracardiac structures—especially the AS and adjacent structures—obtained by ICE is optimal (Figure 3).21 The ease of use and, of course, the reliability of information obtained during the procedure mean ICE has taken the place of TEE. However, the catheters are not reusable and the increased cost this entails is a logical drawback. Furthermore, ICE use in pediatric patients is limited due to the catheter size (9 F), although series with similar but larger catheters (11 F) in patients weighing <15 kg and with mean age 3.1 years, have been reported.25
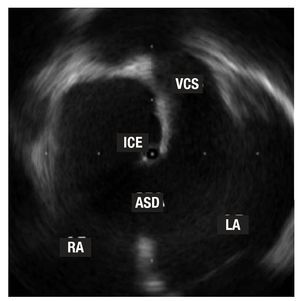
Figure 3. The tip of the intracardiac echocardiography (ICE) catheter is seen in the left atrium (LA) thru a large ostium secundum-type atrial septal defect (ASD). Note the vena cava superior (VCS) opening in the right atrium (RA).
Discrepancies in ASD size on using 3 different references—TEE, ICE, and stretching balloon—are well-known.11,15 Stretching balloon measurement—strongly defended by some teams—produces overstretching of the AS until a radiologic waist is visible in the balloon at the level of the defect, which is later measured to quantify its maximum diameter. It is frequently observed that ASD rims are extremely flimsy, and overstretching can magnify defect size and lead to choosing too large a device or generate complications like arrhythmias or pericardial bleeding.26,27
In our series, measures obtained by TEE provide defect diameters inferior to those obtained by ICE. Several possible justified of these findings exist:
- TEE may not always visualize rims clearly; inferior or posterior rims are better defined by ICE21,28
- ICE can be introduced into the defect and cross it towards the LA permitting the examination of defect geometry from the interior with more perspectives. Septal defects are not usually perfectly circular or symmetrical, and diameters can vary significantly according to the observation plane
Intracardiac echocardiography measures defects in 2 standard planes (longitudinal and transversal) and verification of landmark rims around defects provides enough reliable information to choose an adequately sized device, generally 1-2 mm greater than the maximum defect measurement, in order to ensure correct, firm deployment over the AS.28
Although numerous ASD closure devices are currently commercially available, Amplatzer occluders have proven more versatile and more efficient than other devices. They are easy to implant, can be retrieved in cases of malpositioning, and present a minimal complication rate: all this makes them the simplest and most efficient devices for the interventional cardiologist.28-31 In our series, we found no serious complications (device embolization, erosion, systemic embolism), and in only 3 patients did we record paroxystic atrial fibrillation in the weeks following; this responded to medical treatment. At 6 months, all patients were evaluated by transthoracic echocardiography and in selected patients (with very large devices or devices not centered in the AS) TEE was used. Although residual shunting thru the device is still documented in the first hours post-implantation (determined by color Doppler), the Amplatzer design, integrating polyester inserts on the interior, facilitates complete defect occlusion in the following weeks or months in practically all patients.31
Limitations of the Study
This study is limited in that it deals with a single center, is retrospective, and reflects a small sample.
CONCLUSIONS
In our experience, ICE provides excellent anatomic information on AS and nearby structures in patients with septal defects. The ease of use and added comfort for patients—avoiding sedation or general anesthesia with orotracheal intubation—make the technique an attractive, safe alternative to TEE during ASD occluding device implantation.
SEE EDITORIAL ON PAGES 451-3
ABBREVIATIONS
AS: atrial septum
ASD: atrial septal defect
ASO: Amplatzer septal occluder
ICE: intracardiac echocardiography PFO: patent foramen ovale
TEE: transesophageal echocardiography
Correspondence:
Dr. F. Hernández.
Unidad de Hemodinámica y Cardiología Intervencionista. Hospital 12 de Octubre.
Avda Córdoba s/n. 28041 Madrid. España.
E-mail: fhernandezh@medynet.com
Received March 22, 2007.
Accepted for publication January 4, 2008.