Keywords
INTRODUCTION
Aortic intramural hematoma (IMH) is one of several conditions included in the acute aortic syndrome (AAS).1,2 In morphologic terms it is characterized by hemorrhage into the media layer of the aortic wall in the absence of an intimal-medial tear, which means that is no communication with the aortic lumen. Some authors have suggested that IMH is the result of spontaneous rupture of the aortic vasa vasorum. The frequency of IMH in patients with clinically suspected AAS ranges from 5% to 20% of cases.2-4
As is the case similar to classic aortic dissection, IMH is distinguished into two types according to the Stanford classification: type A, when there is involvement of the ascending aorta, and type B, when the ascending aorta is not affected.
It is currently accepted that treatment for type A IMH is essentially surgical, whereas type B can be initially treated with medical therapy, reserving surgery or stent placement for cases with added complications. Mortality associated with IMH in the first months is not negligible; the main independent predictive factors for death are aortic diameter >50 mm and ascending aorta involvement.5
There are still some uncertainties as to the natural history of this disease and the most appropriate treatment for affected patients. Classically, it is advised to avoid anticoagulation in patients with an AAS mainly for two reasons: to limit the dissecting progression of the hematoma and to avoid cardiac tamponade in patients with pericardial effusion.
The aim of the present study is to describe the clinical and radiological characteristics, as well as the evolution of a subgroup of patients with IMH who received anticoagulant treatment during hospitalization.
METHODS
All patients admitted to our hospital with a diagnosis of AAS from January 2000 to December 2005 were prospectively assessed. Among these patients, we selected all those who had an IMH and had received anticoagulant treatment during hospitalization. Demographic information as well as clinical, radiological, and follow-up data were compiled.
The diagnosis of AAS was established by computed tomography (CT) scanning with and without contrast enhancement. The subsequent follow-up imaging studies were done with CT and/or magnetic resonance (MR).
All the patients studied received medical treatment directed toward strict control of blood pressure (BP ≤120/80 mm Hg), which included beta blockers in all cases, except when there were contraindications for this treatment.
RESULTS
During the study period, 95 patients with a diagnosis of AAS were admitted to our center. Twelve (25.6%) of these patients presented IMH (4 type A and 8 type B). Three patients with IMH who received anticoagulant treatment during hospitalization (one with type A IMH and 2 with type B IMH) are the subject of this report.
The clinical characteristics and main demographic information of the patients with IMH who received anticoagulation are described below and presented in Table.
Case 1
A 79-year-old man with poorly controlled hypertension despite pharmacological treatment and chronic atrial fibrillation. The patient's clinical history included an episode of arterial embolism in the upper right limb 5 years previously, which had been treated with oral anticoagulation. He was admitted for intense pain of sudden onset in the interscapular region, which radiated to the abdomen and was accompanied by profuse sweating and dizziness. Computed tomography performed in the emergency unit identified a type B IMH that extended from the origin of the left subclavian artery to the aortoiliac bifurcation and affected the first few centimeters of the left common iliac artery (Figure 1). At the time of admittance, the patient was treated with labetalol and nitroprusside by perfusion, and anticoagulation was discontinued. Following discontinuation of this treatment, the patient presented a new episode of arterial embolism in the left upper limb that required embolectomy. For this reason, anticoagulation was established, initially with sodium heparin and later with acenocoumarol.
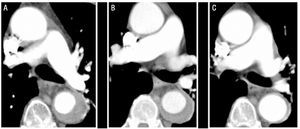
Figure 1.Radiological evolution of type B aortic intramural hematoma (IMH) in patient 1. Serial CT slices at the pulmonary artery bifurcation show progressive decrease in the thickness of the lesion and final resolution. A: IMH thickness at the time of diagnosis. B: thickness at first CT follow-up (2 weeks of evolution). C: IMH thickness at 2 months.
Case 2
A 72-year-old man with hypertension of lengthy evolution under pharmacological treatment, and a history of chronic atrial fibrillation, for which he was receiving digoxin and acenocoumarol. He came to our center for oppressive middle chest pain of sudden onset that radiated to the back. The initial CT scan showed a type A IMH with involvement of the ascending aorta, aortic arch, and descending aorta to the origin of the superior mesenteric artery. Anticoagulation was discontinued. During hospitalization, the patient presented an episode of loss of consciousness with left hemiparesis, from which he recovered without sequelae. Cerebral CT showed no signs of ischemia or intracranial hemorrhages; hence, the episode was interpreted as a transient ischemic attack of embolic origin. Due to this complication, anticoagulant treatment with sodium heparin in perfusion followed by acenocoumarol was restarted during hospitalization.
Case 3
A 73-year-old man, smoker, with chronic renal failure and a history of hypertension of lengthy evolution; he had chronic atrial fibrillation and was receiving antiplatelet therapy. The patient came to our hospital for severe interscapular pain radiating to both flanks. The initial CT scan established the diagnosis of type B intramural hematoma confined to the descending thoracic aorta. In the distal segment of the hematoma, a tear-shaped image (ulcer-like projection) was seen within the hematoma (Figure 2). Although the patient had never had an episode of embolism, anticoagulant treatment was started at admission because of his high embolic risk profile.
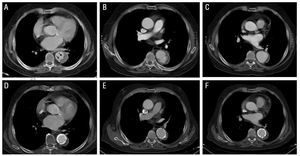
Figure 2. Radiological evolution of type B aortic intramural hematoma (IMH) in patient 3. Upper panel: images at diagnosis (A) and during follow-up (B and C). A: observe the tear-shaped image (ulcer-like projection) within the IMH (arrows). B: localized dissection during follow-up (2 months later). C: progressive dilation of the aortic diameter. Lower panel: images at 5 months following diagnosis (D-F). Progressive dilation of the hematoma dictates implantation of two stents.
Radiological Follow-Up
The maximum aortic diameter and the maximum IMH diameter at the time of diagnosis, as well as the evolution of the lesion in each patient are shown in Table.
Over the radiological follow-up, all patients showed a gradual decrease in IMH thickness. In patients 1 and 2, the maximum diameter of the IMH at the time of diagnosis was 17.6 and 7 mm, respectively. At the 2-week CT follow-up study the diameters were 13 and 4.6 mm, respectively.
In patient 3, IMH thickness was 14 mm on the initial CT and 11.5 mm on the follow-up scan. In addition, the patient presented a tear-shaped image at the distal end of the IMH, which measured 4×6 mm at the time of diagnosis. Some authors have interpreted this as a sign indicating an aortic ulcer (ulcer-like projection). Following hospital discharge, the patient developed a localized dissection with subsequent progressive dilation of the affected aortic segment (pseudoaneurysm) that required implantation of 2 stents (Excluder 34×10 mm and 40×10 mm). The subsequent evolution was favorable (Figure 2).
DISCUSSION
Over the study period studied, 3 patients with IMH received anticoagulant treatment. Even though IMH is a hemorrhagic lesion, the clinical and morphological evolution was favorable in this subgroup of patients, with progressive regression of lesion size in follow-up radiological assessments, despite the fact that anticoagulation was given. Patient 3 developed a complication during follow-up in a segment of the aorta in which the initial lesion may have been a penetrating aortic ulcer (PAU). Nonetheless, the hematoma initially underwent changes similar to those seen in the other two patients, with a gradual decrease in diameter during the acute phase. The role anticoagulation might have played in the pathogenesis and evolution to a pseudoaneurysm is difficult to establish.
There are no definitive data in the scientific literature regarding the use of anticoagulation in patients with AAS, although discontinuation of this treatment is common practice. It is likely that anticoagulation does not modify the natural course of IMH. Three arguments support this hypothesis: a) given that hemostasis does not have a major role in the pathogenesis of the lesion, it is unlikely that it would have an impact on its resolution; b) IMH is a condition confined to the interior of the aortic wall and is not in contact with the vessel lumen, thus it is improbable that the anticoagulant would penetrate the lesion; and c) epidemiological studies have not shown a higher incidence of IMH in patients with congenital or acquired disturbances of the components of hemostasis.
We conclude that in situations where anticoagulation is necessary (patients with a high risk of embolism) this treatment can be maintained, based on the results of this study in which anticoagulation did not seem to have an impact on the clinical or morphological evolution of IMH. Naturally, further studies with larger patient series are required to draw definitive conclusions.
Correspondence: Dr. I. Vilacosta.
Instituto Cardiovascular. Hospital Universitario de San Carlos.
Martín Lagos, s/n. 28040 Madrid. España.
E-mail: ivilac@medynet.com
Received May 3, 2006.
Accepted for publication July 11, 2006.