Drug-eluting stents (DES) have significantly reduced the rate of stent restenosis (SR) and the need for repeat interventional procedures. The presence of stent fractures (SF) with the appearance of SR and/or DES thrombosis, particularly with sirolimus-eluting DES (Cypher®), has recently been reported.1,2 However, the actual incidence of SF is uncertain, and SFs can be hard to diagnose by angiography alone.
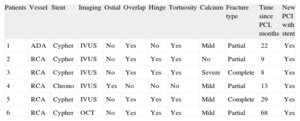
We describe the incidence of SF confirmed by intravascular imaging in a patient population angiographically assessed due to suspected SR. A total of 355 SR-type lesions were treated at our site between January 2007 and June 2012: 197 (55%) were conventional stents and 158 (45%) were DES. Intravascular ultrasound or optical coherence tomography was used in 169 (48%) lesions. The incidence of SF confirmed by intravascular imaging was 3.6% (6 of 169 lesions). The characteristics of the SFs are shown in the Table.
Characteristics of Lesions With Stent Fractures
| Patients | Vessel | Stent | Imaging | Ostial | Overlap | Hinge | Tortuosity | Calcium | Fracture type | Time since PCI, months | New PCI with stent |
| 1 | ADA | Cypher | IVUS | No | Yes | No | Yes | Mild | Partial | 22 | Yes |
| 2 | RCA | Cypher | IVUS | No | Yes | Yes | Yes | No | Partial | 9 | Yes |
| 3 | RCA | Cypher | IVUS | No | Yes | Yes | Yes | Severe | Complete | 8 | Yes |
| 4 | RCA | Chrono | IVUS | Yes | No | No | No | Mild | Partial | 13 | Yes |
| 5 | RCA | Cypher | IVUS | No | Yes | Yes | Yes | Mild | Complete | 29 | Yes |
| 6 | RCA | Cypher | OCT | No | Yes | Yes | Yes | Mild | Partial | 68 | Yes |
ADA, anterior descending artery; IVUS, intravascular ultrasound; OCT, optical coherence tomography; PCI, percutaneous coronary intervention; RCA, right coronary artery.
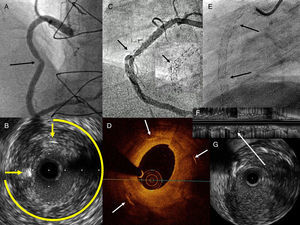
SF was radiologically visible in only 1 of the 6 cases identified. In all others, it was suspected due to the presence of focal SR in a tortuous area or a hinge point. On occasion, the SR was not angiographically significant, but the intravascular ultrasound showed considerable focal hyperplasia and the absence of struts in an arch >270° (Figs. A and B). The intravascular image was analyzed frame by frame to identify suspected areas and to delimit the presence or absence of struts (Figs. C and D). All 6 patients underwent repeat percutaneous coronary intervention with stent implantation in the SR area. Further clinical follow-up and noninvasive tests showed no new events (median, 9 [3] months).
A: Stent restenosis in the middle right coronary artery (arrow). B: Intravascular ultrasound image of A, with abundant concentric hyperplasia, only 2 visible struts (arrows), and a 270° arch with no struts. C: Radiologic image of overlapping stents in the right coronary, with focal restenosis in the angle area and broad image of stent distortion (arrow). D: Optical coherence tomography image of C; abundant concentric hyperplasia and only 3 struts (arrows) very far apart. E: Radiologic image of the right coronary artery; stents from the ostium to the distal segment by chronic occlusion; the arrows indicate 2 areas of complete fracture with physical separation of the struts. F: Longitudinal intravascular ultrasound image of E; the arrow indicates the fracture without struts. G: Transverse intravascular ultrasound image of E; absence of struts.
SF may be diagnosed by radiography (contrast-free imaging) or intravascular imaging, such as intravascular ultrasound or optical coherence tomography. Classifications have been described for both methods, based on the clear separation of struts or the absence of struts in one or more coronary segments.3,4 Factors that favor the appearance of SF have also been reported, such as the presence of calcium, tortuous arteries, and the degree of artery torsion during the cardiac cycle.
The actual incidence of SF is uncertain. Various studies report an incidence of 0.84% to 8.4%, and on many occasions SF is detected during investigations for SR.5 SF can go unnoticed if not radiologically obvious or if the vessel is not investigated using intracoronary imaging. The most common intravascular findings are the absence of struts in a broad area of the vessel circumference and the presence of abundant hyperplasia in the same segment.6 At times, it may be difficult to differentiate between incomplete SF and stent deformation (pronounced curves, postdilation of lateral branches, calcified lesions), which causes strut separation and asymmetry, but it is not difficult to detect the absence of struts. If the fracture is complete, total disappearance of struts is observed in various consecutive images (Figs. E-G).
The pathogenic mechanisms of SR or SF thrombosis are probably related to the lower amount of drug dispensed in the fracture area and to greater mechanical aggression from the fractured struts, as both of these factors cause smooth muscle cell proliferation and abnormal endothelialization.1,5
Some studies have shown that certain sirolimus-eluting DES (Cypher®) cause a greater number of SF because of their closed-cell design and the use of stainless steel, a material of lower flexibility and conformability than new cobalt, chromium, or platinum alloys. Right coronary or saphenous sites are more common, as are long stents, overlapping stents, and tortuous lesions or hinge points.5 This series reviewed previous percutaneous coronary intervention procedures in cases of overlap, to rule out an actual separation between stents, and confirmed correct stent position during release.
This observational register has several limitations. Angiographic follow-up was carried out only in patients with suspected SR based on clinical symptoms compatible with this diagnosis or ischemia observed in noninvasive tests. Intravascular ultrasound or optical coherence tomography was used in half the patients, and thus the number of undiagnosed SF could have been even higher. Furthermore, optical coherence tomography is still used infrequently, although its imaging resolution is better able to identify struts than intravascular ultrasound.
In conclusion, SF diagnosis using intravascular imaging techniques is extremely accurate and is superior to diagnosis by radiologic imaging alone. This finding has prognostic implications, because SF increases the risk of SR and thrombosis. Some sirolimus-eluting DES (Cypher®) have a higher incidence of SF than other DES.
.