Although clinical guidelines recommend invasive management in non–ST-segment elevation myocardial infarction (NSTEMI), this strategy is underused in frail elderly patients in the real world. Furthermore, these patients are underrepresented in clinical trials and therefore the evidence is scarce. Our hypothesis is that an invasive strategy will improve prognosis in elderly frail patients with NSTEMI.
MethodsThis will be a prospective, multicenter, randomized trial, in which the conservative and invasive strategies will be compared in patients meeting all of the following inclusion criteria: NSTEMI diagnosis, age ≥ 70 years, and frailty defined by a category ≥ 4 in the Clinical Frailty Scale. Participants will be randomized to an invasive (coronary angiogram and revascularization if anatomically amenable) or conservative (medical treatment and coronary angiogram only if persistent clinical instability) strategy. The primary endpoint will be the number of days alive out of hospital during the first year. The coprimary endpoint will be the time until the first cardiac event (cardiac death, reinfarction or postdischarge revascularization). We estimate a sample size of 178 patients (89 per arm), considering an increase of 20% in the proportion of days alive out of hospital with the invasive management.
ResultsThe results of this study will add important knowledge to inform the management of frail elderly patients hospitalized with NSTEMI.
ConclusionsWe hypothesize that the invasive strategy will improve outcomes in frail elderly patients with NSTEMI. If this is confirmed, frailty status should not dissuade physicians from implementing an invasive management strategy. Clinical trial registration: URL: http://www.clinicaltrials.gov.Identifier: NCT03208153.
Keywords
Frailty is defined as a physiologic state of decreased resistance to stressors that results from decreased physiologic reserves of multiple systems and causes vulnerability to adverse outcomes.1 Acute coronary syndromes imply a major stressor for frail patients. Indeed, frailty predicts short- and long-term mortality after acute coronary syndrome.2–7 Furthermore, among geriatric conditions (namely physical disability, instrumental disability, cognitive impairment, and comorbidities), frailty along with comorbidities capture most of the prognostic information.4,6
There is a lack of evidence on the optimal management of frail patients with non–ST-elevation acute myocardial infarction (NSTEMI). Although clinical practice guidelines recommend early invasive management based on the results of clinical trials, frail patients are underrepresented in these trials. Therefore, the role of the invasive strategy in frail patients is currently uncertain. We hypothesize that a routine invasive strategy will improve outcomes in frail patients. The aim of the present trial is to evaluate the efficacy and safety of a routine invasive strategy in increasing the number of days alive at home and in improving cardiovascular outcomes during the first year after NSTEMI.
METHODSStudy DesignThis is an investigator-mediated, prospective, multicenter, randomized clinical trial comparing an invasive vs a noninvasive strategy in patients with NSTEMI, aged ≥ 70 years with frailty. Patients will be eligible for inclusion if they fulfill all 3 of the following inclusion criteria: a) NSTEMI, defined by acute chest pain, absence of persistent ST-segment elevation in the presence of interpretable repolarization and troponin elevation (according to the local laboratory troponin assay); b) age ≥ 70 years; c) frailty criteria defined by ≥ 4 points on the Clinical Frailty Scale ().8 The exclusion criteria will be the following: a) prior known nonrevascularizable coronary artery disease; b) significant concomitant nonischemic heart disease (ie, severe heart valve disease, cardiomyopathy); c) inability to understand/sign informed consent (patients or relatives); d) life expectancy < 12 months.
In addition to the defined inclusion and exclusion criteria, the attending cardiologist must believe that the participation of the patient in the study is reasonable. Reasons for considering participation inappropriate might be either the consideration by the attending cardiologist of the need for an invasive management strategy or any clinical factor making invasive management not an option.
This study was registered at ClinicalTrials.gov (Identifier: NCT03208153).
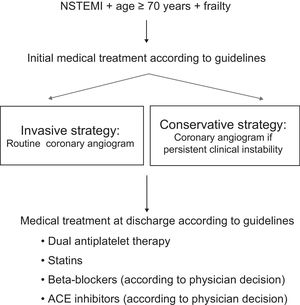
ManagementPatients will be randomized within 48hours of admission to undergo coronary angiography and coronary revascularization if deemed appropriate during the index hospitalization, or to be treated with medical therapy. Patients will be assigned to both treatment groups using a computer-generated randomization scheme to allocate participants in a 1:1 ratio. The randomization will be via a web site where the process will be concealed from the researchers until the interventions are assigned. Medical treatment at admission and discharge will be optimized according to the guidelines in both study arms.
The Figure shows the algorithm of the study. The invasive group will undergo coronary angiography within 72hours of admission. When percutaneous coronary intervention is performed, the type of stent implanted will left to the judgement of the treating cardiologist, although stents with proven safety using short-term antiplatelet treatment are recommended to reduce the potential bleeding risk of this population. In contrast, patients allocated to the noninvasive group will receive medical therapy only, although cardiac catheterization will be allowed if there is poor in-hospital outcome due to documented recurrent ischemia (chest pain plus either electrocardiogram changes or troponin re-elevation) or hemodynamic instability during the index admission. According to a previous a trial, the estimated crossover rate is 24%.9 Predischarge left ventricular ejection fraction will be measured in all patients by echocardiography. The standard period of dual antiplatelet therapy will be 1 year in both study arms, although in patients at high bleeding risk (in the judgement of the treating physician) or in need of oral anticoagulation therapy, 1 antiplatelet drug may be withdrawn after the first month. Detailed clinical and treatment data will be collected at admission and during hospitalization (Table 1 and Table 2).
Variables Collected at Admission
| Hospitalization: Coronary unit, cardiology ward, internal medicine ward, geriatric ward, other |
| Inclusion criteria (all of them): Non–ST-segment elevation acute myocardial infarction, age (≥ 70 years), frailty criteria (Clinical Frailty Scale ≥ 4) |
| Exclusion criteria (none of them): Prior known nonrevascularizable coronary artery disease, significant concomitant nonischemic heart disease, unable to understand/sign informed consent (patients or relatives), life expectancy < 12 months |
| Demographic data: Sex, weight, height |
| Risk factors: Diabetes (insulin, metformin, sulphonylureas, other), hypertension (number of drugs), dyslipidemia (statins, fibrates, ezetimibe, other), smoking, documented peripheral artery disease, prior stroke, prior renal failure (glomerular filtration rate < 60 mL/min, dialysis) |
| Prior history of heart disease: Acute myocardial infarction, documented ischemic heart disease with coronary angiogram, percutaneous coronary intervention, coronary artery bypass graft, admission for heart failure, atrial fibrillation, cardiac pacemaker or defibrillator, detailed prior drug treatment |
| Comorbidities: Prior hospitalization for bleeding, neoplasm, liver disease, chronic treatment with nonsteroidal anti-inflammatory drugs, dementia, depression (drugs), alcohol intake, total number of drugs |
| Admission data related to non–ST-elevation myocardial infarction: Time of pain onset, time of admission, ≥ 2 pain episodes in the previous 24hours, electrocardiogram findings, blood pressure, heart rate, Killip class |
Variables Collected During Hospitalization
| Coronary angiography (if performed): Access (radial/femoral), contrast volume, dominance, culprit artery, initial TIMI flow, number of vessels with significant stenosis, left main disease, SYNTAX Score, revascularization procedure, EuroSCORE II |
| Percutaneous coronary intervention: Number or treated vessels, direct stenting, complete revascularization, number of stents, type of stent, final TIMI flow. |
| Echocardiography: Left ventricular ejection fraction, mitral regurgitation, pericardial effusion |
| Blood tests: Hemoglobin (admission and minimum), white blood cell count (admission), creatinine (admission and maximum), glucose (admission and maximum), LDL-C, HDL-C, triglycerides |
| Drug treatments during hospitalization |
| Clinical complications: Death, reinfarction, recurrent angina, AV block, ventricular tachycardia, ventricular fibrillation, atrial fibrillation, mechanical complication, bleeding (Bleeding Academic Research Consortium criteria), delirium, stroke |
| Drug treatments at discharge |
AV, atrioventricular; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TIMI, Thrombolysis In Myocardial Infarction.
A systematic and comprehensive geriatric evaluation will be performed prior to discharge through interview with the patient and/or family/caregivers and referring to the patient's status prior to admission, as follows: a) Functional capacity for basic activities of daily living will be assessed by the Barthel Index.10 This is an ordinal scale with a total score of 0 to 100, where the intermediate ranges help evaluate the different degrees of dependency: total (0-20), severe (21-40), moderate (41-60), light (61-90), and independent (> 90). b) Instrumental activities will be evaluated with the Lawton-Brody Index.11c) Cognitive status will be evaluated with the Pfeiffer test.12d) Previous frailty will be assessed, in addition to the Rockwood scale, by the FRAIL scale, which includes evaluation of fatigue, resistance, ambulation, concomitant diseases, and weight loss.13e) After the acute clinical phase (once the patient is stabilized), the Short Physical Performance Battery will be carried out.14 This test includes: i) balance in 3 positions (feet together, semitandem and tandem), ii) walking speed (4 meters along), and iii) get up and sit in a chair 5 times. The SPPB total score is the summation of the 3 subtests, ranging from 0 (worst) to 12. A score below 10 indicates frailty and an increased risk of disability and falls. f) To assess comorbidity, the Charlson index will be applied, with a maximum score of 37 points.15 The number of medications with chronic prescription taken by the patient before admission will be also recorded. g) Nutritional risk assessment will be carried out with the Mini Nutritional Assessment-Short Form,16 whose value ranges from 0 to 14 points; scores below 11 identify patients at risk of malnutrition. h) Quality of life will be analyzed by the EQ 5D 5L test.17
EndpointsThe primary endpoint will be the number of days alive out of hospital during the first year. The coprimary endpoint will be the time to the first occurrence of 1 of the events included in the combined endpoint (cardiovascular death, myocardial infarction or postdischarge revascularization). Secondary endpoints will include time to occurrence of all-cause death, myocardial infarction, rehospitalization for cardiac and extracardiac causes, bleeding episodes, and stroke. Only bleeding type ≥ 2 according to the Bleeding Academic Research Consortium definition will be considered.18 The occurrence of procedure-related myocardial infarction will be evaluated by measuring troponins 12hours after revascularization. Procedure-related infarction will be defined by troponin elevation > 5 x 99th percentile after percutaneous coronary intervention and > 10 x 99th percentile after coronary surgery, plus data suggestive of acute ischemia according to clinical symptoms, electrocardiogram, angiography or imaging. Functional capacity (Barthel index), instrumental activities (Lawson-Brody index), cognitive capacity (Pfeiffer) and quality of life (EQ 5D5L) will be reassessed at 6 months, also as secondary endpoints. Follow-up will include a clinical visit or telephone contact at 3 months, 1 year, and yearly until 3 years.
Study CommitteesThis is an investigator-driven initiative under the auspices of the Spanish Society of Cardiology and the working groups of Interventional Cardiology and Geriatric Cardiology. A steering committee will be responsible for overseeing the scientific and operational aspects of the study. Patients and investigators will not be masked to treatment allocation, but clinical events will be assessed by a blinded clinical event committee to prevent bias. Likewise, a data safety monitoring board, unaware of the patients’ treatment allocation, will be responsible for making recommendations to the steering committee regarding endpoints and any potential significant patient safety-related observations.
Sample SizeThere are no data on the optimal management of frail patients with NSTEMI. In a previous study in elderly patients with NSTEMI and comorbidities, patients who underwent a conservative strategy remained alive out of hospital for a mean of 273 days (standard deviation 123 days) during the first year after discharge.9 Assuming that frail patients might have a fairly similar profile and considering an increase of 20% in the proportion of days alive out of hospital (55 days) with an invasive strategy, we estimate a sample size of 178 patients (89 per arm) with an estimated power of 80%, 2-sided alpha level of .05, and 10% of losses of follow-up. The achievement of the sample size will require a multicenter approach. We estimate at least 10 participating hospitals.
Statistical AnalysisAll statistical comparisons will be made under the intention-to-treat principle. Results will be presented as frequencies or mean (standard deviation), as appropriate. Between-group comparisons will be performed using the t test or Fisher exact test.
Patient follow-up will be censored at the time of death or at the end of the study. The primary endpoint, as a continuous variable, will be compared between the 2 groups using the ANOVA test. Regarding the coprimary endpoint and secondary endpoints, the effect of the invasive strategy on the clinical events will be depicted by a Kaplan-Meier method and assessed using a Cox regression model. The hazard ratio and 95% confidence interval will be calculated. A prespecified subgroup analysis according to comorbidities (Charlson index) will be conducted.
A 2-sided P value of < .05 will be considered statistically significant.
DISCUSSIONThe prevalence of frailty in older patients admitted for acute coronary syndrome ranges between 27% and 34%.4,19 Its presence is associated with mortality risk, both during admission and after discharge.2–7 This might be partly due to the underuse of guideline-recommended therapies. Clinical guidelines recommend a routine invasive strategy in NSTEMI.20 Nevertheless, invasive management is underused in frail and comorbid patients.2,21–23 This policy might derive from the perception of certain risks linked to invasive procedures in this particularly vulnerable population, such as bleeding, contrast nephropathy, or challenging revascularization procedures (for example, on severe calcified lesions). However, with the use of the radial approach, prophylaxis measures for contrast nephropathy and new revascularization devices, concerns about the invasive approach do not seem justified. Nevertheless, the perception of the lack of benefit due to the unmodifiable poor prognosis conferred by frailty status per se might still be an argument.
A few studies have addressed the role of invasive strategies in elderly patients. In a study by Savonitto et al.,24 a routine invasive strategy was not statistically superior to a selective invasive strategy in elderly patients with NSTEMI, but the study was underpowered due to the small sample size. The After Eighty randomized trial was a proper-sized study that included patients older than 80 years with NSTEMI and demonstrated the benefit of an invasive strategy in reducing the composite endpoint of death or cardiovascular events at 1.5 years.25 It is worth noting that no patient underwent cardiac catheterization under any circumstance in the conservative arm of that study. Furthermore, only 23% of the potential candidates for inclusion were finally randomized, suggesting a bias toward lower-risk patients. Recently, the MOSCA randomized trial evaluated the efficacy of an invasive strategy in elderly patients with NSTEMI and comorbidities.9 Although this was a small trial, there were no differences between the invasive and conservative strategies. In an exploratory nonprespecified analysis, the invasive strategy reduced the probability of death or ischemic events at 3 months. This benefit, nonetheless, vanished at 2.5-years’ follow-up. There is no solid information regarding frailty. In fact, frail patients have usually been excluded from randomized clinical trials. The TRILOGY ACS trial (TaRgeted platelet Inhibition to cLarify the Optimal strateGy to medicallY manage Acute Coronary Syndromes), for example, included a remarkably low rate (4.7%) of frail patients.5 An observational study suggests that invasive management could be of benefit in frail patients with NSTEMI.26 This hypothesis must be validated in a dedicated randomized trial.
Some relationship was observed between age and bleeding risk in patients under dual antiplatelet therapy.27 This relationship was not found with frailty.5 As a whole, physicians tend to avoid the new potent antiplatelet agents in elderly or frail patients based on the increased bleeding risk. Related to this fact, interventional cardiologists have been reluctant to use drug-eluting stents due to concerns about the need of longer duration of dual antiplatelet therapy. Furthermore, some frail patients require oral anticoagulation for chronic atrial fibrillation. However, new stent designs allow a short dual antiplatelet period and might constitute an excellent tool.28
Finally, while most studies mainly focus on death, myocardial infarction, stroke, need for revascularization or rehospitalization, patients are also willing to recover an independent life and return to their usual place of residence. The presence of geriatric syndromes (including frailty, cognitive impairment, severe dependence, and depression) is not only associated with worse clinical outcomes but also with a greater risk of functional decline and need for new social help, which means an increased level of dependence. This has a substantial impact on quality of life and psychological wellbeing but also frequently becomes a heavy social and economic burden for patients and families. Therefore, one of the real challenges in the management of acute coronary syndrome in very old patients is the prevention of dependence. In this regard, the use of new outcomes especially addressed to measure level of independence and quality of life is especially important.29
LimitationsThe estimation of the sample size is speculative in part, since there are no previous data on the prognostic impact of the management strategy in frail patients with NSTEMI. Therefore, we had to use the information from our previous study in older patients with comorbidities and NSTEMI to estimate the sample size, even though frailty was not measured in the study.9
CONCLUSIONSThe optimal management strategy for frail patients with NSTEMI is unknown. No trial has been designed for this particular population so far. We hypothesize that the invasive strategy will improve outcomes in frail elderly patients with NSTEMI. If this is confirmed, frailty status should not dissuade physicians from implementing an invasive management.
FUNDINGThis work was supported by grants from Spain's Ministry of Economy and Competitiveness through the Carlos III Health Institute: FIS 17/01736, FIS 17/00899 and FIS 15/00837, FEDER; CIBER-CV 16/11/00420, Madrid, Spain. It is also supported by the SCReN-Spanish Clinical Research Network (PT13/0002/0031; PT17/0017/0003) from the National R+D+I Plan of the Institute of Health Carlos III (Ministry of Economy and Competitiveness: Cofinanced by European Regional Development Fund “A way to make Europe”), for monitoring the study
CONFLICTS OF INTERESTE. Abu-Assi is Associate Editor of Revista Española de Cardiología.
H. Bueno reports grants and personal fees from AstraZeneca, personal fees from Daiichi Sankyo, personal fees from Eli Lilly, personal fees from Bayer, personal fees from Sanofi, during the conduct of the study; personal fees from Novartis, personal fees from BMS-Pfizer, from Servier, outside the submitted work.
J. Núñez reports personal fees from Novartis, personal fees from Vifor, personal fees from Abbott, personal fees from Rovi, personal fees from Boehringer Ingelheim, personal fees from Novo Nordisk, outside the submitted work.
M. Sanmartín reports personal fees from Bayer, Boehringer Ingelheim, and Astra, outside the submitted work.
- –
Clinical practice guidelines recommend an invasive strategy in NSTEMI, based on the results of randomized clinical trials.
- –
Elderly frail patients are underrepresented in randomized clinical trials.
- –
Therefore, the optimal management strategy for elderly frail patients with NSTEMI remains unknown.
- –
To our knowledge, this is the first randomized clinical trial comparing invasive and conservative strategies in elderly frail patients with NSTEMI.
- –
Previous randomized trials have focused on elderly patients without taking into account frailty status.
- –
The results of the trial will provide relevant information for the management of this challenging population.
The authors thank Marta Peiró, M. Dolores Iglesias and Mireia Hernández, for monitoring the study.