Iron deficiency (ID), with or without anemia, is a prevalent comorbidity in patients with chronic heart failure that confers a worse outcome.1,2 There are no data on the prevalence of ID development or its associated factors in acute coronary syndrome (ACS).
Here, we present a descriptive analysis of patients admitted to our center for ACS. This analysis forms part of a prospective registry of patients with ACS that will be used, once patient inclusion and follow-up have been completed, to analyze the prognostic implication of this comorbidity in this clinical setting. The following patients were excluded: those who refused to provide informed consent, those referred to another center during admission, those who died in the first 5 days after the ACS, and those who had major bleeding or received treatment with blood derivatives or iron. Inflammatory parameters (ultrasensitive C-reactive protein and interleukin-6 [IL 6]) and iron metabolism data were determined at 5 and 30 days. In line with the international consensus, ID was defined as ferritin < 100 ng/mL or as ferritin < 800 ng/mL if transferrin saturation was < 20%. After patient inclusion, data were collected on demographic and clinical variables potentially involved in ID development.
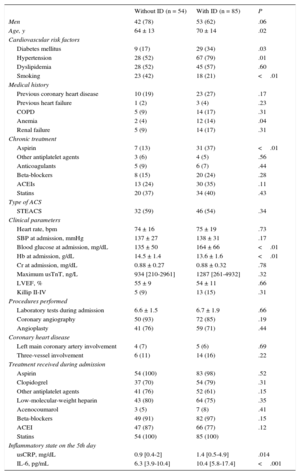
A total of 139 patients (age, 67 ± 14 years; 32% women) were included between November 2012 and June 2014. Of these, 85 (61%) had ID and 39 (28%) had anemia. These rates decreased to 54% and 23% among the 119 patients whose analytical determinations were performed at 30 days. Patients with ID had higher blood glucose, lower hemoglobin (Hb), and higher C-reactive protein and IL-6 concentrations (P ≤ .01) (Table). No differences were found in the proportion of ACS with ST elevation, distribution of coronary lesions, and treatment received during hospitalization.
Clinical and Treatment Characteristics of Patients With Acute Coronary Syndrome With and Without Iron Deficiency
| Without ID (n = 54) | With ID (n = 85) | P | |
|---|---|---|---|
| Men | 42 (78) | 53 (62) | .06 |
| Age, y | 64 ± 13 | 70 ± 14 | .02 |
| Cardiovascular risk factors | |||
| Diabetes mellitus | 9 (17) | 29 (34) | .03 |
| Hypertension | 28 (52) | 67 (79) | .01 |
| Dyslipidemia | 28 (52) | 45 (57) | .60 |
| Smoking | 23 (42) | 18 (21) | <.01 |
| Medical history | |||
| Previous coronary heart disease | 10 (19) | 23 (27) | .17 |
| Previous heart failure | 1 (2) | 3 (4) | .23 |
| COPD | 5 (9) | 14 (17) | .31 |
| Anemia | 2 (4) | 12 (14) | .04 |
| Renal failure | 5 (9) | 14 (17) | .31 |
| Chronic treatment | |||
| Aspirin | 7 (13) | 31 (37) | <.01 |
| Other antiplatelet agents | 3 (6) | 4 (5) | .56 |
| Anticoagulants | 5 (9) | 6 (7) | .44 |
| Beta-blockers | 8 (15) | 20 (24) | .28 |
| ACEIs | 13 (24) | 30 (35) | .11 |
| Statins | 20 (37) | 34 (40) | .43 |
| Type of ACS | |||
| STEACS | 32 (59) | 46 (54) | .34 |
| Clinical parameters | |||
| Heart rate, bpm | 74 ± 16 | 75 ± 19 | .73 |
| SBP at admission, mmHg | 137 ± 27 | 138 ± 31 | .17 |
| Blood glucose at admission, mg/dL | 135 ± 50 | 164 ± 66 | <.01 |
| Hb at admission, g/dL | 14.5 ± 1.4 | 13.6 ± 1.6 | <.01 |
| Cr at admission, mg/dL | 0.88 ± 0.27 | 0.88 ± 0.32 | .78 |
| Maximum usTnT, ng/L | 934 [210-2961] | 1287 [261-4932] | .32 |
| LVEF, % | 55 ± 9 | 54 ± 11 | .66 |
| Killip II-IV | 5 (9) | 13 (15) | .31 |
| Procedures performed | |||
| Laboratory tests during admission | 6.6 ± 1.5 | 6.7 ± 1.9 | .66 |
| Coronary angiography | 50 (93) | 72 (85) | .19 |
| Angioplasty | 41 (76) | 59 (71) | .44 |
| Coronary heart disease | |||
| Left main coronary artery involvement | 4 (7) | 5 (6) | .69 |
| Three-vessel involvement | 6 (11) | 14 (16) | .22 |
| Treatment received during admission | |||
| Aspirin | 54 (100) | 83 (98) | .52 |
| Clopidogrel | 37 (70) | 54 (79) | .31 |
| Other antiplatelet agents | 41 (76) | 52 (61) | .15 |
| Low-molecular-weight heparin | 43 (80) | 64 (75) | .35 |
| Acenocoumarol | 3 (5) | 7 (8) | .41 |
| Beta-blockers | 49 (91) | 82 (97) | .15 |
| ACEI | 47 (87) | 66 (77) | .12 |
| Statins | 54 (100) | 85 (100) | |
| Inflammatory state on the 5th day | |||
| usCRP, mg/dL | 0.9 [0.4-2] | 1.4 [0.5-4.9] | .014 |
| IL-6, pg/mL | 6.3 [3.9-10.4] | 10.4 [5.8-17.4] | <.001 |
ACS, acute coronary syndrome; ACEIs, angiotensin-converting enzyme inhibitors; COPD, chronic obstructive pulmonary disease; Cr, creatinine; Hb, hemoglobin; ID, iron deficiency; IL-6, interleukin 6; LVEF, left ventricular ejection fraction; renal failure, glomerular filtration < 60 mL/min/1.73 m2; SBP, systolic blood pressure; STEACS, ST elevation acute coronary syndrome; usCRP, ultrasensitive C-reactive protein; usTnT, ultrasensitive troponin T.
The data are expressed as n (%), mean ± standard deviation, or median [interquartile range].
Multivariable logistic regression analysis showed that IL-6 (P = .011), Hb on admission (P = .001), and pretreatment with aspirin (P = .021) were independent predictors of ID.
The present study showed a high ID prevalence in ACS (61%). This high prevalence also persisted in more than half of the patients 30 days after the coronary event. Both findings are novel and only comparable in the context of ischemic heart disease with the series of Jankowska et al,3 which reported the presence of ID in 48% of patients with stable coronary artery disease undergoing cardiac surgery. The association between ID and ACS could have unknown prognostic implications in quality of life and long-term functional capacity.
The small sample size and short follow-up do not permit clarification of the prognostic impact of ID on ACS, limiting the clinical implications of our findings until the conclusion of patient inclusion and follow-up. Neither is analysis possible of the pathophysiological mechanisms causing ID in the setting of ACS. However, the independent association between ID and chronic aspirin therapy, low Hb values, and a heightened inflammatory state (high levels of IL-6 and C-reactive protein) is alarming.
More specifically, the relationship between ID and inflammatory status has been seen in patients with advanced chronic heart failure.4 In contrast, a previous study by our group found that patients with a more heightened inflammatory status admitted for ACS had a higher probability of anemia development during hospitalization.5 Our data are in accordance with those of Huang et al,6 who showed a significant association between low serum iron and high IL-6 concentrations in patients with ST elevation ACS. As is well known, inflammation plays a role in atherosclerotic plaque formation, and inflammatory status is more pronounced at the time of plaque rupture. It seems plausible, although still speculative, that coronary heart disease (and, more specifically, its decompensation) and ID would show common etiopathogenic mechanisms related to this inflammatory status, beyond a possible etiopathogenic association per se between ID and ACS.
The most likely cause of the association between ID and chronic aspirin therapy is undetected chronic gastrointestinal bleeding. However, it is unclear whether the possible development of ID indicates a need to modify the antiplatelet strategy in these patients.
In conclusion, ID is a prevalent and persistent condition in ACS associated with chronic antiplatelet therapy, anemia, and a heightened inflammatory status, with unknown prognostic implications.
FUNDINGThe research reported in this publication was supported by the Sociedad Catalana de Cardiología (Catalan Society of Cardiology) with the Servier 2012 grant.
CONFLICTS OF INTERESTJ. Comín-Colet was a member of the executive committees of the FAIR-HF and CONFIRM-HF trials (both sponsored by Vifor Pharma Ltd), and has received honoraria for conferences from Vifor Pharma Ltd.