Iron deficiency (ID) is a prevalent condition in patients with ischemic heart disease and heart failure. Little is known about the impact of ID on exercise capacity and quality of life (QoL) in the recovery phase after an acute coronary syndrome (ACS).
MethodsIron status and its impact on exercise capacity and QoL were prospectively evaluated in 244 patients 30 days after the ACS. QoL was assessed by the standard EuroQoL-5 dimensions, EuroQoL visual analogue scale, and Heart-QoL questionnaires. Exercise capacity was analyzed by treadmill/6-minute walk tests. The effect of ID on cardiovascular mortality and readmission rate was also investigated.
ResultsA total of 46% of the patients had ID. These patients had lower exercise times (366±162 vs 462±155seconds; P<.001), metabolic consumption rates (7.9±2.9 vs 9.3±2.6 METS; P=.003), and EuroQoL-5 dimensions (0.76±0.25 vs 0.84±0.16), visual analogue scale (66±16 vs 72±17), and Heart-QoL (1.9±0.6 vs 2.2±0.6) scores (P<.05). ID independently predicted lower exercise times (OR, 2.9; 95%CI, 1.1-7.6; P=.023) and worse QoL (OR, 1.9; 95%CI, 1.1-3.3; P<.001) but had no effect on cardiovascular morbidity or mortality.
ConclusionsID, a prevalent condition in ACS patients, results in a poorer mid-term functional recovery, as measured by exercise capacity and QoL.
Keywords
Iron is an essential micronutrient for oxygen transportation and storage, cardiac and skeletal muscle metabolism, and protein synthesis and degradation.1 In recent years, iron deficiency (ID) has been characterized as a common comorbidity in some cardiovascular diseases and has been associated with worse clinical outcomes and impaired exercise capacity in chronic heart failure.2,3 The prognostic impact of ID on heart failure patients is independent of its association with anemia; furthermore, ID reversion with intravenous iron improves functional capacity independently of any increase in hemoglobin (Hb) levels.4
Few studies have analyzed the prevalence and clinical determinants of ID in patients with ischemic heart disease, and the available results are focused on its chronic phase.5–8 In 1 of these series, ID was associated with increased mortality in patients with stable ischemic heart disease undergoing coronary angioplasty; however, ID was not analyzed separately, as it was always associated with anemia.7
To date, the impact of ID on the clinical outcomes of patients presenting with an acute coronary syndrome (ACS) is unknown. We hypothesized that ID precludes adequate mid-term functional recovery after an ACS, as measured by exercise capacity and quality of life (QoL) scores, independently of any association with anemia. For exploratory purposes, we further described the effect of ID on mid-term cardiovascular morbidity and mortality.
METHODSStudy Population and RecruitmentThis study was approved by the local ethics committee for clinical research and was conducted according to the principles laid down in the Declaration of Helsinki.
Consecutive patients admitted to our hospital with ACS were prospectively considered for inclusion. All participants gave written informed consent at the time of inclusion. We excluded patients whose ID status could not be determined at day 5 after the ACS and those who were discharged or died before study inclusion. We also excluded patients receiving iron therapy or blood products during hospital admission.
Clinical, biological, echocardiographic, and demographic variables, as well as chronic drug therapy were collected at admission. During hospitalization, the distribution of the coronary artery disease (whenever available) and other therapeutic interventions were also incorporated into the study database.
Analysis of Iron DeficiencyHb levels were measured on admission. On day 5 after the ACS event, a complete hematological evaluation was undertaken, including repeated Hb levels, mean corpuscular volume, iron indices (ie, serum ferritin, transferrin, transferrin saturation, and serum iron) and inflammatory parameters (ie, high-sensitivity C-reactive protein [CRP] and interleukin-6 [IL-6]). The decision not to perform these tests in the early acute phase of the ACS was based on the assumption that labile IL-6/CRP levels and the variability and time-dependency of antithrombotic agents administered within the first 48hours of the ACS would preclude homogeneous characterization of inflammatory and hematological status.
Iron deficiency was defined according to Kidney Disease Outcomes Quality Initiative guidelines as ferritin levels of < 100 ng/mL or as a percentage of transferrin saturation (defined as serum iron (μg/dL)/[serum transferrin (mg/dL)×1.25])<20% when ferritin is < 800 ng/mL.9,10 This dual definition takes into account both functional and absolute aspects of ID. Anemia was defined as Hb levels < 13g/dL (men) or < 12g/dL (women), according to World Health Organization guidelines.11
Follow-up: Quality of Life and Exercise Capacity AssessmentPatient follow-up at 30 days after the ACS index event took place at the outpatient clinic. The QoL questionnaires and a treadmill exercise test using the Bruce protocol (or, if not feasible, a 6-minute walk test) were administered and the comprehensive hematological evaluation was repeated. The latter 2 tests were omitted as necessary; minimum follow-up consisted of registration of clinical status by telephone.
We used 2 different QoL questionnaires: the generic European QoL-5 dimensions (EQ-5D) plus the visual analogue scale questionnaire and the more specific Heart-QoL test.12–14 The EQ-5D questionnaire is a self-reported questionnaire of the patient's health-related QoL in 5 dimensions of daily life (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression). The scoring system in this questionnaire categorizes 5 levels of QoL impairment (no problems, slight problems, moderate problems, severe problems, extreme problems).12 On the visual analogue scale questionnaire, the patient self-rates his or her health-related QoL status on a 20-cm vertical line. In this study, the reference chosen to contextualize the scores obtained by our study population on this scale was the median value (78 points) of the results of the visual analogue scale questionnaire reported for the overall Spanish population.13 The 14-item Heart-QoL questionnaire specifically addresses patients with ischemic heart disease. Patients who report a worse physical and emotional status than their peers also achieve lower Heart-QoL questionnaire scores.14
Statistical AnalysisFor the sample size calculation, we took as a reference previous data analyzing QoL in patients with coronary artery disease and a previous myocardial infarction (a score of 0.80 on the EQ-5D questionnaire) and also considered the prevalence of ID in this population.6,15 On the basis of these data, we hypothesized that ACS-ID patients would score 0.75±0.1 on the EQ-5D questionnaire.15 We admitted an α risk of 0.05 and a β risk of 0.20 in a 2-tailed test, and we estimated a 5% percentage of patients lost to follow-up. As a result, the sample size calculation rendered an estimated sample of 63 cases and 63 controls (total sample size: a minimum of 132 consecutive patients). Further patient inclusion was allowed to provide additional descriptive data regarding the effect of ID on cardiovascular morbidity and mortality.
Data are expressed as mean±standard deviation for data with normal distribution and median plus 25-75 percentiles for data with nonnormal distribution. Categorical variables are expressed as frequency and percentage. Clinical differences between the ID and non-ID groups were analyzed with the Student t test, Mann-Whitney U test, the chi-square test, or the Fisher exact test as appropriate.
We used a logistic regression model to establish the clinical determinants of ID. A multivariate linear regression model was used to analyze the effect of ID and the other tested variables on exercise capacity. In both models, the clinical determinants of ID reaching a P value of < .10 in the univariate model were incorporated into a multivariate analysis. Backward modeling was used to assess the independent association between the clinical variables, ID, and exercise capacity. Each variable was removed, one by one, if its exclusion did not significantly modify the likelihood ratio statistics of the model. When removal of any variable changed the estimated parameters of the remaining variables by > 15%, it was considered a confounding effect and the variable was retained in the model regardless of its statistical significance. Calibration was assessed by the Hosmer and Lemeshow test and diagnostic capacity by the area under the receiver operating characteristic curve.
IL-6 and CRP were log transformed (log-IL-6 and log-CRP). To check for linearity, the log-IL-6 was smoothed in a generalized additive model and the P value for nonlinear effects was calculated.
A 2-sided P value of <.05 was considered statistically significant. The statistical analysis was performed with the SPSS 19.0 software package.
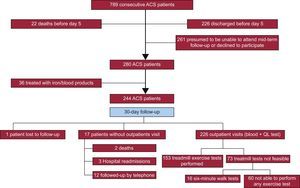
RESULTSStudy PopulationA total of 789 patients with a diagnosis of ACS were admitted to our hospital from November 2012 through October 2015, of whom 244 were included in our study. The reasons for noninclusion were early transfer to another institution (266 patients) and likelihood of suboptimal mid-term follow-up (261 patients including tourists, individuals with a language barrier, patients with impaired cognition, and patients who refused to participate). The patient inclusion flowchart is shown in Figure 1.
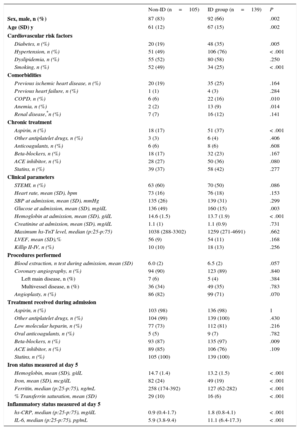
ID was diagnosed in 139 (57%) patients at day 5, and anemia was diagnosed in 50 of these patients (20% of the total study population). Iron deficiency was significantly associated with old age and most cardiovascular risk factors and comorbidities (P ≤ .01, except for dyslipidemia and renal disease), as well as with chronic aspirin intake (Table 1). Prior heart failure was underrepresented in our series (only 4 patients), and lacked statistical significance.
Clinical Characteristics of Patients With and Without Iron Deficiency
| Non-ID (n=105) | ID group (n=139) | P | |
|---|---|---|---|
| Sex, male, n (%) | 87 (83) | 92 (66) | .002 |
| Age (SD) y | 61 (12) | 67 (15) | .002 |
| Cardiovascular risk factors | |||
| Diabetes, n (%) | 20 (19) | 48 (35) | .005 |
| Hypertension, n (%) | 51 (49) | 106 (76) | < .001 |
| Dyslipidemia, n (%) | 55 (52) | 80 (58) | .250 |
| Smoking, n (%) | 52 (49) | 34 (25) | < .001 |
| Comorbidities | |||
| Previous ischemic heart disease, n (%) | 20 (19) | 35 (25) | .164 |
| Previous heart failure, n (%) | 1 (1) | 4 (3) | .284 |
| COPD, n (%) | 6 (6) | 22 (16) | .010 |
| Anemia, n (%) | 2 (2) | 13 (9) | .014 |
| Renal disease,*n (%) | 7 (7) | 16 (12) | .141 |
| Chronic treatment | |||
| Aspirin, n (%) | 18 (17) | 51 (37) | < .001 |
| Other antiplatelet drugs, n (%) | 3 (3) | 6 (4) | .406 |
| Anticoagulants, n (%) | 6 (6) | 8 (6) | .608 |
| Beta-blockers, n (%) | 18 (17) | 32 (23) | .167 |
| ACE inhibitor, n (%) | 28 (27) | 50 (36) | .080 |
| Statins, n (%) | 39 (37) | 58 (42) | .277 |
| Clinical parameters | |||
| STEMI, n (%) | 63 (60) | 70 (50) | .086 |
| Heart rate, mean (SD), bpm | 73 (16) | 76 (18) | .153 |
| SBP at admission, mean (SD), mmHg | 135 (26) | 139 (31) | .299 |
| Glucose at admission, mean (SD), mg/dL | 136 (49) | 160 (15) | .003 |
| Hemoglobin at admission, mean (SD), g/dL | 14.6 (1.5) | 13.7 (1.9) | < .001 |
| Creatinine at admission, mean (SD), mg/dL | 1.1 (1) | 1.1 (0.9) | .731 |
| Maximum hs-TnT level, median (p:25-p:75) | 1038 (288-3302) | 1259 (271-4691) | .662 |
| LVEF, mean (SD),% | 56 (9) | 54 (11) | .168 |
| Killip II-IV, n (%) | 10 (10) | 18 (13) | .256 |
| Procedures performed | |||
| Blood extraction, n test during admission, mean (SD) | 6.0 (2) | 6.5 (2) | .057 |
| Coronary angiography, n (%) | 94 (90) | 123 (89) | .840 |
| Left main disease, n (%) | 7 (6) | 5 (4) | .384 |
| Multivessel disease, n (%) | 36 (34) | 49 (35) | .783 |
| Angioplasty, n (%) | 86 (82) | 99 (71) | .070 |
| Treatment received during admission | |||
| Aspirin, n (%) | 103 (98) | 136 (98) | 1 |
| Other antiplatelet drugs, n (%) | 104 (99) | 139 (100) | .430 |
| Low molecular heparin, n (%) | 77 (73) | 112 (81) | .216 |
| Oral anticoagulants, n (%) | 5 (5) | 9 (7) | .782 |
| Beta-blockers, n (%) | 93 (87) | 135 (97) | .009 |
| ACE inhibitor, n (%) | 89 (85) | 106 (76) | .109 |
| Statins, n (%) | 105 (100) | 139 (100) | |
| Iron status measured at day 5 | |||
| Hemoglobin, mean (SD), g/dL | 14.7 (1.4) | 13.2 (1.5) | < .001 |
| Iron, mean (SD), mcg/dL | 82 (24) | 49 (19) | < .001 |
| Ferritin, median (p:25-p:75), ng/mL | 258 (174-392) | 127 (62-282) | < .001 |
| % Transferrin saturation, mean (SD) | 29 (10) | 16 (6) | < .001 |
| Inflammatory status measured at day 5 | |||
| hs-CRP, median (p:25-p:75), mg/dL | 0.9 (0.4-1.7) | 1.8 (0.8-4.1) | < .001 |
| IL-6, median (p:25-p:75), pg/mL | 5.9 (3.8-9.4) | 11.1 (6.4-17.3) | < .001 |
ACE inhibitor, angiotensin-converting enzyme inhibitor; bpm, beats per minute; COPD, chronic obstructive pulmonary disease; hs-CRP, high-sensitivity C-reactive protein; hs-TnT, high-sensitivity troponin T; ID, iron deficiency; IL-6, interleukin-6; LVEF, left ventricular ejection fraction; SBP, systolic blood pressure; SD, standard deviation; STEMI, ST-segment elevation myocardial infarction.
We observed no differences in the incidence of hemorrhagic complications between the ID and non-ID groups. Compared with the non-ID group, patients with ID were older, had lower Hb levels, and higher IL-6/CRP levels (P<.001; Table 1). A linear correlation between higher IL-6 levels and a higher risk of developing ID was established (P value for nonlinear term=.461).
Consistent with our preliminary observation, the multivariate logistic regression model confirmed that higher IL-6 levels (odds ratio [OR], 1.048 per each 1 pg/mL increase; 95% confidence interval [95%CI], 1.013-1.084; P=.007) and prior aspirin intake (OR, 3.254; 95%CI, 1.373-7.716; P=.007) were independently associated with ID.
Follow-up: Iron deficiency, Exercise Capacity, and Quality of LifeID persisted in 102 (46%) of the 226 ACS patients tested at day 30; follow-up was limited in the remaining 18 patients (Figure 1). At this mid-term follow-up, the ID group still had lower Hb levels than the non-ID group (P<.01). Only 1 of the 244 patients was lost during follow-up and 5 patients did not participate in any part of the 30-day follow-up visit due to death or hospital readmission (Figure 1). Overall, there had been 7 major acute cardiovascular events at day 30, including 2 deaths and 5 hospital readmissions (4 due to heart failure and 1 due to myocardial reinfarction). This low incidence of major acute cardiovascular events precluded assessment of significant differences between groups, with no preliminary differences between the ID and non-ID groups in our series.
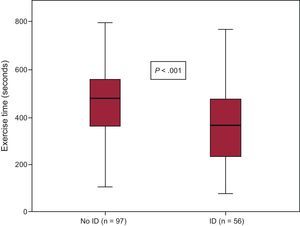
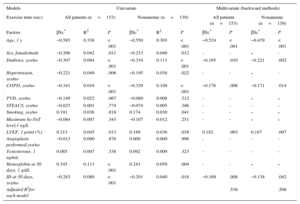
Iron Deficiency and Exercise CapacitySixty (27%) of the 226 patients did not undergo any exercise tests, due to severe osteoarthritis, peripheral artery disease, loss of balance, and/or suboptimal adaptation to the treadmill. Of the remaining patients (Figure 1), 153 completed the treadmill test and 13 did the 6-minute walk test instead. Iron deficiency was 41% in this group and 66% in those who could not perform any exercise test (P<.001). In the treadmill group, patients with persistent ID at day 30 had lower exercise capacity, as measured by total exercise time and a lower rate of metabolic consumption than those without ID at follow-up (7.9±2.9 vs 9.3±2.6 METS; P=.003 and 366±162 vs 462±155seconds; P<.001, respectively) (Figure 2). These differences were not influenced by the proportion of patients with and without ID who were taking beta-blockers (93% vs 92% respectively; P=.80). Patients with ID who did the 6-minute walk test also walked a shorter distance than patients without ID (277 vs 423 meters respectively; P=.009). When adjusted for other comorbidities, ID was significantly associated with a lower exercise capacity in the multivariate linear regression model (P=.008), as were older age, diabetes, chronic pulmonary disease, and low left ventricular ejection fraction (Table 2). Importantly, the association between ID and lower exercise capacity was accompanied by a significant increase in the R2 coefficient in this multivariate regression model (from 0.510 to 0.538) and it remained statistically significant when nonanemic patients were independently analyzed (P=.048; Table 2).
Exercise time achieved by the ID and non-ID patient groups. Within each box, the middle horizontal line corresponds to the median, the lower limit to the first quartile, and the upper limit to the third quartile. The whiskers represent the 95% confidence interval of the mean. ID, iron deficiency.
Exercise Capacity on Treadmill Test. Univariate and Multivariate Linear Regression Models for the Analysis of Demographics and Clinical Factors Related to Exercise in all Patients and in Nonanemic Patients
| Models | Univariate | Multivariate (backward methods) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exercise time (sec) | All patients (n=153) | Nonanemic (n=139) | All patients (n=153) | Nonanemic (n=139) | ||||||
| Factors | βSc* | R2 | P | βSc* | R2 | P | βSc* | P | βSc* | P |
| Age, 1 y | −0.585 | 0.338 | < .001 | −0.550 | 0.303 | < .001 | −0.524 | < .001 | −0.479 | < .001 |
| Sex, female/male | −0.206 | 0.042 | .011 | −0.213 | 0.046 | .012 | - | - | - | - |
| Diabetes, yes/no | −0.307 | 0.094 | < .001 | −0.334 | 0.111 | < .001 | −0.165 | .010 | −0.221 | .002 |
| Hypertension, yes/no | −0.221 | 0.049 | .006 | −0.195 | 0.038 | .022 | - | - | - | - |
| COPD, yes/no | −0.341 | 0.016 | < .001 | −0.329 | 0.109 | < .001 | −0.176 | .006 | −0.171 | .014 |
| PVD, yes/no | −0.149 | 0.022 | .067 | −0.089 | 0.008 | .312 | - | - | - | - |
| STEACS, yes/no | −0.023 | 0.001 | .774 | −0.074 | 0.005 | .386 | - | - | - | - |
| Smoking, yes/no | 0.191 | 0.036 | .018 | 0.174 | 0.030 | .041 | - | - | - | - |
| Maximum hs-TnT level,1 ng/L | −0.084 | 0.007 | .343 | −0.107 | 0.012 | .251 | - | - | - | - |
| LVEF, 1 point (%) | 0.213 | 0.045 | .013 | 0.189 | 0.036 | .038 | 0.182 | .003 | 0.187 | .007 |
| Angioplasty performed,yes/no | −0.013 | 0.000 | .878 | 0.000 | 0.000 | .996 | - | - | - | - |
| Testosterone, 1 ng/mL | 0.085 | 0.007 | .338 | 0.092 | 0.009 | .323 | - | - | - | - |
| Hemoglobin at 30 days, 1 g/dL | 0.345 | 0.113 | < .001 | 0.243 | 0.059 | .004 | - | - | - | - |
| ID at 30 days, yes/no | −0.283 | 0.080 | < .001 | −0.201 | 0.040 | .018 | −0.169 | .008 | −0.138 | .042 |
| Adjusted R2for each model | .538 | .508 | ||||||||
COPD, chronic obstructive pulmonary disease; GF, glomerular filtration; hs-TnT level, high-sensitivity troponin T; ID, iron deficiency; LVEF, left ventricular ejection fraction; PVD, peripheral vascular disease; STEACS, ST-segment elevation acute coronary syndrome.
Similarly, when exercise capacity was analyzed as a binary variable, and with the median exercise time (418seconds) being taken as the reference, most patients with ID had exercise times below this median value and ID was associated with impaired functional capacity (ie, exercise time < 418seconds) in both the univariate (OR, 2.8; 95%CI, 1.4-5.5; P=.004) and the multivariate (OR, 2.9; 95%CI, 1.1-7.6; P=.023) analyses.
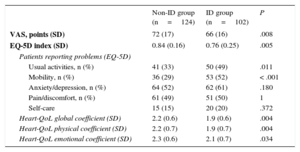
Iron Deficiency and Quality of LifeA total of 226 patients underwent blood testing and completed the QoL questionnaires at day 30. The ID group had lower scores than the non-ID group on the EQ-5D questionnaire (P=.005), visual analogue scale questionnaire (P=.008), and Heart-QoL questionnaires (P=.004) (Table 3). Iron deficiency mainly influenced the ‘mobility’ and ‘usual activities’ domains in the EQ-5D questionnaire. On the visual analogue scale questionnaire, 75% of the ID group scored below the preliminary 78-point cutoff value compared with 49% of the non-ID group (P<.001). Similarly, 60% of the patients with ID scored below the median value (2.21 points) in the Heart-QoL questionnaire, compared with only 42% of the non-ID group (P=.009). After adjustment by age, sex, anemia, chronic obstructive pulmonary disease, renal failure, hypertension, and diabetes, ID remained an independent predictor of impaired QoL according to the visual analogue scale questionnaire (OR, 3.021; 95%CI, 1.672-5.457; P<.001) and Heart-QoL questionnaires (OR, 1.9; 95%CI, 1.079-3.348; P<.001).
Quality of Life at 30 Days
| Non-ID group (n=124) | ID group (n=102) | P | |
|---|---|---|---|
| VAS, points (SD) | 72 (17) | 66 (16) | .008 |
| EQ-5D index (SD) | 0.84 (0.16) | 0.76 (0.25) | .005 |
| Patients reporting problems (EQ-5D) | |||
| Usual activities, n (%) | 41 (33) | 50 (49) | .011 |
| Mobility, n (%) | 36 (29) | 53 (52) | < .001 |
| Anxiety/depression, n (%) | 64 (52) | 62 (61) | .180 |
| Pain/discomfort, n (%) | 61 (49) | 51 (50) | 1 |
| Self-care | 15 (15) | 20 (20) | .372 |
| Heart-QoL global coefficient (SD) | 2.2 (0.6) | 1.9 (0.6) | .004 |
| Heart-QoL physical coefficient (SD) | 2.2 (0.7) | 1.9 (0.7) | .004 |
| Heart-QoL emotional coefficient (SD) | 2.3 (0.6) | 2.1 (0.7) | .034 |
EQ-5D, European quality of life-5 dimensions questionnaire; Heart-QoL, heart disease health-related quality of life questionnaire; ID, iron deficiency; SD, standard deviation; VAS, visual analogue scale.
Values are expressed as mean (SD) or n (%).
Statistical significance was set at P<.05.
The present study identified ID as a major determinant of impaired functional capacity (P<.01) and QoL (P<.01) after an ACS, independently of the presence or absence of anemia. Our study defines ID as a highly prevalent underlying comorbidity in the setting of an ACS that could constitute a potential pharmacological target to support functional recovery after the ACS index event. In this regard, the role of iron repletion deserves further investigation. The very low death and reinfarction rates observed in our series precluded confirmation of ID as a clinical predictor of major cardiovascular morbidity and mortality after an ACS.
Pathophysiological AspectsThe definition of ID originally derives from reported series of patients with chronic kidney disease, who frequently have an indication for iron repletion. Recently, ID has been characterized in other chronic diseases, such as rheumatoid arthritis and other inflammatory diseases, heart failure, and ischemic heart disease.
Identification of ID is challenging in the pathological processes that are accompanied by inflammation, such as an ACS. In this scenario, ID is suggested when ferritin and/or the serum iron levels (which have a controversial prognostic impact in a number of cardiovascular diseases) are not used as the sole criterion; the definition of ID also includes the degree of transferrin saturation (‘multi-marker’ definition).9,10,16–19
ID is a highly prevalent condition in patients with cardiovascular disease, especially in stable and acute coronary artery disease and chronic heart failure.5,6,20–23 There is no clear evidence on the mechanisms involved in the development of ID in cardiac patients. The present study corroborated an earlier report that chronic use of aspirin and proinflammatory status (as demonstrated by increased CRP/IL-6 levels) are independent determinants of ID in ACS patients.6 Chronic gastrointestinal bleeding and common upstream inflammatory pathways shared by coronary artery disease (and specifically its destabilization) and the ID pathological process, respectively, may account for such associations.16,24,25 Proinflammatory status reduces both the absorption/availability of iron (ID) and participates in the ‘destabilization’ of the coronary atherosclerotic plaque causing the ACS event.26–34
Iron Deficiency, Functional Capacity, and Quality of LifeID entails a decreased oxidative capacity of the skeletal muscle and an increased reliance on carbohydrates as the substrate for energy, thereby causing impaired endurance. This principle is independent of the association between ID and anemia.35,36 The presence of ID has been associated with worse physical performance and lower oxygen uptake (VO2 max consumption) in both young athletes and sedentary women without anemia.37–39 In addition, in heart failure patients, ID produces impaired exercise capacity.2 Our findings indicate that ID also jeopardizes aerobic work capacity in the mid-term after an ACS. Interestingly, low Hb levels were not independently associated with ID in the multivariate analyses (data not shown) and ID was also associated with a decreased exercise capacity in patients who were not anemic. These observations emphasize the primary role of ID in the patient's functional recovery after the ACS event, beyond the association with anemia.
In the present study, patients with ID reported poorer QoL, essentially linked to perceived limitations in mobility and physical activity capacities. Functional impairments drive important effects of self-perceived health status.17,40 In light of our results, this assumption can also be extrapolated to the clinical setting of the mid-term recovery phase after an ACS.
Iron Deficiency: A Therapeutic Target After an Acute Coronary Syndrome?Many nonmodifiable factors such as sex, age, education, and marital/occupational status may affect self-perceived functional capacity and QoL.41 The identification of a potentially modifiable variable such as ID might provide a pharmacological target in the search for optimal functional recovery after an ACS event. Correction of ID improves both QoL and exercise performance in heart failure patients.42,40,43 Determining whether ID reversion will drive a significant mid-term improvement in QoL and exercise capacity after an ACS will require further investigation.
Study LimitationsBecause ID and inflammatory parameters were assessed on day 5 after the ACS, patients who died in the acute phase of the ACS were not represented in this study, thus constituting a potential selection bias. The decision to delay iron/inflammatory status determination was made to prevent the results from being influenced by hypoxia time, ischemia-reperfusion phenomenon, and the antithrombotic treatment administered during the initial phases of the ACS.44
The standardized beta coefficient featuring the impact of ID on the patient's functional recovery, although consistent with that reported in heart failure patients, has to be considered somewhat low.3,21 Similarly, the R2 value of 0.54, although higher than that reported in patients with heart failure and ID, could be considered somewhat modest.21,45
The significant number of patients who declined to participate or were excluded due to mid-term follow-up concerns (261 patients) may jeopardize the representativeness of the study sample. In addition, no exercise test could be performed due to mobility problems in one fourth of our patients. We consider this limitation a natural consequence of the phenomenon of progressive aging of ACS patients in the western countries.
The present study lacks statistical power to demonstrate a prognostic effect of ID on cardiovascular outcome after an ACS. Data regarding cardiovascular morbidity and mortality are provided in this study for descriptive and exploratory purposes only.
CONCLUSIONSID is a highly prevalent underlying condition in the ACS setting, and persists in the mid-term. The persistence of ID significantly compromises the exercise capacity and the QoL of ACS patients, independently of any association with anemia. Given its potential reversion by means of iron repletion, ID may offer a pharmacological target in ACS patients, which could support optimal functional recovery after a coronary event. The long-term impact of ID (and its treatment) on major cardiovascular mortality and morbidity in this setting is yet to be determined.
CONFLICTS OF INTERESTJ. Comín-Colet was a member of the FAIR-HF steering committee and the CONFIRM-HF trial (sponsored both by Vifor Pharma Ltd.), and has received honoraria for speaking from Vifor Pharma Ltd. All other authors have no conflicts to declare.
- -
ID is a highly prevalent underlying condition in ACS patients.
- -
Prior use of aspirin, low Hb levels, and proinflammatory status are associated with the development of ID in these patients.
- -
Little is known about the influence of ID on patients’ clinical and functional outcome after an ACS, since this prognostic information has not been previously reported.
- -
Our study revealed that ID is associated with both impaired functional capacity and lower QoL in ACS patients, regardless of the presence or absence of anemia.
- -
Given its potential reversion by means of iron repletion, ID might constitute a pharmacological target in this clinical scenario.
Research reported in this publication was supported by the Catalan Society of Cardiology under a Servier award 2012.