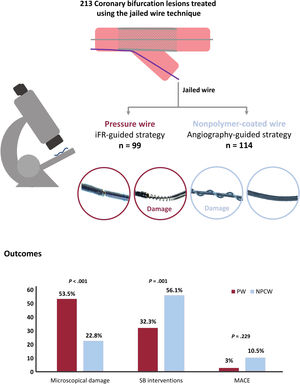
The use of a pressure wire as a jailed wire to evaluate side branch results during provisional stenting seems feasible. However, safety concerns exist due to the mechanical damage of the wire and the lack of prospective data evaluating the prognosis of patients treated using this technique. This study sought to evaluate the structural damage of the pressure wire in patients treated using the jailed pressure wire technique and to assess mid-term clinical outcomes.
MethodsWe enrolled 99 patients with single bifurcation lesions and provisional stenting as the strategy of choice. A jailed pressure wire was used to guide side branch intervention according to the instantaneous wave-free ratio (iFR). A total of 114 patients and the respective nonpolymer-coated jailed wires were used as historical controls. Guidewire damage was evaluated by stereomicroscopy. The primary endpoint was significant microscopic damage. Major adverse cardiac events were evaluated at 2-year follow-up.
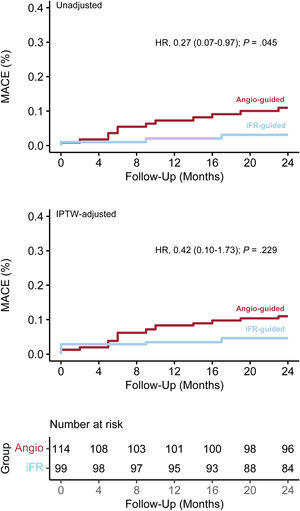
ResultsSignificant microscopic damage was more frequent in pressure wires than in nonpolymer-coated wires (53.5% vs 22.8%, P<.001). There were no fractures in either group. There were fewer side branch interventions in the pressure wire group (postdilation/kissing balloon, 32.3% vs 56.1%, P=.001; stenting, 0.0% vs 2.6%, P=.104). The 2-year rate of major adverse cardiac events was similar between the 2 groups (HRadj, 0.42; 95%CI, 0.10-1.73; P=.229).
ConclusionsPressure wires were less resistant to jailing than conventional nonpolymer-coated wires. Patients treated with iFR-guided provisional stenting required fewer side branch interventions but had similar 2-year clinical outcomes than patients treated with the angiography-guided technique.
Keywords
Coronary bifurcation lesions (CBLs) are present in up to one fifth of patients undergoing percutaneous coronary intervention (PCI) and constitute a challenging subset.1 Although the strategy of choice is subject to debate, provisional stenting (PS) is usually recommended.2 In this approach, the jailed wire technique consists of maintaining a wire in the side branch (SB) during main vessel (MV) stenting. This technique is associated with fewer rates of SB occlusion and facilitates its reopening when needed.3 However, damage or even fracture of the jailed wire may occur during its removal.
A key step in PS is the evaluation of the SB result after MV stenting to decide if additional intervention is needed. Due to the discordance between the angiographic severity and the functional significance of coronary lesions, coronary physiology may facilitate this assessment.5–9 However, SB rewiring with a pressure wire might be particularly troublesome and time-consuming. The jailed pressure wire technique has been proposed to avoid rewiring and to simplify the procedure.10,11 Nevertheless, pressure wires might be more vulnerable to retrieval damage.10
There are limited data regarding the use of the jailed pressure wire technique in the treatment of CBLs and the clinical prognosis of patients treated with this strategy. Thus, we aimed to compare the structural damage of pressure and nonpolymer-coated wires after the jailed wire technique and the mid-term clinical outcomes of patients with CBLs treated by instantaneous wave-free ratio (iFR)-guided and angiography-guided PS.
METHODSDesign and populationWe conducted a single-center, nonrandomized, prospective study enrolling patients undergoing PCI of CBL in whom PS was the strategy of choice. Patients from a previous randomized trial treated with the same technique but using nonpolymer-coated wires were used as historical controls (we excluded 6 participants in whom SB wiring was not possible) (figure 1). The details of this randomized trial have been previously described.4 The study was conducted according to the Declaration of Helsinki and was approved by the local clinical research ethics committee. Written informed consent was obtained from all patients undergoing PCI.
Patients were recruited from April 2017 to June 2020 at Reina Sofia University Hospital, Córdoba, Spain. The following inclusion criteria were established: a) patients aged ≥ 18 years undergoing PCI due to silent angina, stable angina, or acute coronary syndrome; b) the presence of a significant CBL evaluated in 2 orthogonal projections; c) SB size ≥ 2 mm by visual estimation, enough to be protected; d) PS strategy as the initial approach. Major exclusion criteria were: a) cardiogenic shock; b) contraindication to receive prolonged antiplatelet therapy; c) severe calcification requiring rotational atherectomy; d) significant thrombocytopenia (< 10 x 109/L); e) pregnancy; f) life expectancy less than 1 year.
Study endpointsThe primary endpoint was the presence of significant damage in the wires determined by stereomicroscope. Significant damage was defined as the presence of at least moderate microscopic damage as specified in the corresponding section. The secondary endpoint was the 2-year rate of major adverse cardiac events (MACE), defined as a composite of cardiac death, myocardial infarction, and clinically driven target vessel revascularization. Postprocedure myocardial infarction was defined as an elevation of cTn values> 5 times the 99th upper reference limit with evidence of new myocardial ischemia according to the Fourth Universal Definition of Myocardial Infarction.12
ProcedureThe treatment procedure has been previously described in a pilot study.10 Briefly, pressure normalization of the iFR pressure wire (Philips Volcano Verrata and Verrata Plus, Philips, The Netherlands) was performed between the aorta and the MV ostium. Then, the wire was passed distal to the SB lesion and baseline iFR was determined under stable hemodynamic conditions (without vasodilator administration). SB and MV predilation were left to the operator's discretion. Next, the pressure wire was maintained in the SB during MV stent deployment, jailing the wire between the stent and the wall of the MV. At this point, a second SB iFR was quantified using the jailed pressure wire and, subsequently, the wire was retrieved to the MV ostium for drift assessment. When drift exceeded± 0.02, recalibration in the MV, rewiring of the SB and new iFR determination was performed. SB postdilation was performed if iFR was ≤ 0.89. If iFR remained ≤ 0.89 after postdilation, the need for a second stent was considered. Other physiological indexes such as fractional flow reserve (FFR) were not used. Coronary angiography measurements were performed using a quantification software (CAAS system, Pie Medical Imaging, the Netherlands). After the procedure, the jailed pressure wire was cleaned and sent for microscopic analysis.
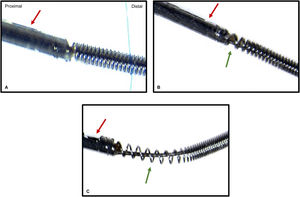
Microscopic studyThe microscopic analysis methodology has previously been described by our group in a randomized controlled trial comparing the microscopic damage of polymer-coated and nonpolymer-coated guidewires after the jailed wire technique.4 In short, we used a SMZ-800 stereomicroscope (Nikon Instruments, United States) with a zoom magnification range of 1.0x to 6.3x. Microphotographs were taken using a DS-Fi1 color camera. Reflected (episcopic) light illumination was used to improve image quality. The examination started with low magnification and was augmented when traces of damage were present. The damage was graded into 5 categories: a) no damage: no loss of integrity over its entire length; b) slight damage: loss of integrity ≤ 2 mm; c) moderate damage: loss of integrity> 2 mm; d) severe damage: changes in the inner part of the wire; e) fracture: discontinuity at some point of the wire. Examples of pressure wire microscopic evaluation are shown in figure 2.
Follow-upPatients were clinically followed up through their visits to the outpatient clinics, telephone calls, and electronic medical records.
Statistical analysisSample size calculation was based on the estimates from our previous works, considering a percentage of significant damage of 45% and 23% in the pressure and nonpolymer-coated wires, respectively.4,10 Accepting an alpha level of 0.05 and a power equal to 80% in a 2-sided test, 71 guidewires were needed in each group to find statistically significant differences for the primary endpoint.
Categorical data are presented as counts (percentages) and continuous data as mean±standard deviation or median [interquartile range]. Between-group comparisons were made using the chi-square test or the Fisher exact test for categorical variables and the Student t-test or the Mann-Whitney U-test for continuous variables. Logistic regression was used to compare microscopic damage between groups. Inverse probability of treatment weighting (IPTW) was used to account for angiographic and technical differences between the 2 groups.13 Propensity scores were calculated using a logistic regression model that included the following covariates: bifurcation location, bifurcation angle, bifurcation type, MV length, MV diameter, SB diameter, severe calcification, severe tortuosity, length of the trapped wire, proximal optimization technique (POT), and SB postdilation (including kissing balloon). Standardized mean differences before and after the weighting were used to evaluate the balance of the groups regarding the covariates. A difference of <10% was considered to indicate good balance. The distributions of the propensity scores before and after weighting were plotted to assess the degree of overlap between the 2 groups. To evaluate the risk of MACE, time-to-event analyses were conducted using Kaplan-Meier and Cox proportional hazards methods, which were also adjusted by IPTW using the same methodology but including POT and the following clinical covariates in the propensity score model: age, sex, left ventricular ejection fraction, diabetes mellitus, clinical presentation, and multivessel disease. Confidence intervals for the IPTW coefficients were obtained using robust sandwich-type variance estimators.14 All tests were 2-tailed and were considered significant when P <.05. Statistical analyses were performed using SPSS software (version 24; IBM Corp, United States) and R software (version 4.0.3; R Foundation for Statistical Computing, Austria).
RESULTSBaseline characteristicsIn all, 99 participants with single CBLs treated with the jailed pressure wire technique (iFR group) were included in the current study. The control group was composed by 114 patients treated with the jailed wire technique using nonpolymer-coated wires. The baseline clinical data of the unweighted study patients were similar between the 2 groups (table 1). There were no significant clinical differences between groups in terms of age, sex, cardiovascular risk factors, previous revascularization, heart failure, or left ventricular ejection fraction. However, more patients in the control group presented with stable angina. Angiographic data are summarized in table 2. The groups were similar regarding the CBL location, MV and SB reference diameter, and the presence of severe tortuosity and calcification. There were significant differences in the presence of multivessel disease, the type of CBL according to the Medina classification, the bifurcation angle and some of the quantitative measurements (MV and SB minimal lumen diameter, lesion length, and diameter stenosis).
Baseline clinical characteristics
| iFR (n=99) | Control (n=114) | P | |
|---|---|---|---|
| Age, y | 64.7±10.2 | 65.9±10.6 | .406 |
| Sex (male) | 77 (77.8) | 82 (71.9) | .328 |
| Presentation | .001 | ||
| Stable angina | 45 (45.5) | 17 (14.9) | |
| Unstable angina | 18 (18.2) | 72 (63.2) | |
| NSTEMI | 24 (24.2) | 5 (4.4) | |
| STEMI | 12 (12.1) | 20 (17.5) | |
| Current smoker | 22 (22.2) | 23 (20.2) | .715 |
| Hypertension | 67 (67.7) | 81 (71.1) | .594 |
| Hypercholesterolemia | 50 (50.5) | 66 (57.9) | .280 |
| Diabetes mellitus | 32 (32.3) | 37 (32.5) | .984 |
| Previous CAD | 16 (16.2) | 10 (8.8) | .100 |
| Previous PCI | 13 (13.1) | 9 (7.9) | .210 |
| Heart failure | 10 (10.1) | 22 (19.3) | .061 |
| LVEF | 61 [53-68] | 60 [53-66] | .374 |
CAD, coronary artery disease; iFR, instantaneous wave-free ratio; LVEF, left ventricular ejection fraction; NSTEMI, non–ST-segment elevation myocardial infarction; STEMI, ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention.
The data are presented as No. (%), mean±standard deviation, or median [interquartile range].
Lesion characteristics
| iFR (n=99) | Control (n=114) | P | |
|---|---|---|---|
| Multivessel disease | 47 (47.5) | 74 (64.9) | .010 |
| Bifurcation location | .334 | ||
| Distal LM | 17 (17.2) | 14 (12.3) | |
| LAD | 58 (58.6) | 63 (55.3) | |
| LCX | 17 (17.2) | 21 (18.4) | |
| RCA | 7 (7.1) | 16 (14.0) | |
| Medina classification | .021 | ||
| 1,1,1 | 22 (22.2) | 46 (40.4) | |
| 1,1,0 | 46 (46.5) | 47 (41.2) | |
| 1,0,1 | 3 (3.0) | 3 (2.6) | |
| 0,1,1 | 5 (5.1) | 4 (3.5) | |
| 1,0,0 | 10 (10.1) | 10 (8.8) | |
| 0,1,0 | 13 (13.1) | 3 (2.6) | |
| 0,0,1 | 0 (0.0) | 1 (0.9) | |
| True bifurcation | 30 (30.3) | 53 (46.5) | .016 |
| Main vessel | |||
| RD, mm | 3.0 [2.7-3.2] | 3.0 [2.8-3.3] | .141 |
| MLD, mm | 0.9±0.5 | 0.6±0.3 | .001 |
| Lesion length, mm | 15.0 [12.7-22.0] | 14.0 [11.0-17.5] | .003 |
| Diameter stenosis, mm | 70.0 [58.3-80.0] | 79.0 [74.0-87.0] | .001 |
| Side branch | |||
| RD, mm | 2.5 [2-0-2.8] | 2.3 [2.1-2.5] | .184 |
| MLD, mm | 2.0 [1-0-2.4] | 1.3 [0.8-2.1] | .004 |
| Lesion length, mm | 5.0 [4.0-7.8] | 7.6 [5.5-9.3] | .004 |
| Diameter stenosis, mm | 11.0 [0.0-50.0] | 43.0 [2.5-64.0] | .005 |
| Bifurcation angle, | 62.9 [43.8-91.7] | 61.5 [45.4-83.3] | .001 |
| Severe tortuosity | 2 (2.0) | 3 (2.7) | .768 |
| Severe calcification | 8 (8.1) | 7 (7.0) | .769 |
iFR, instantaneous wave-free ratio; LM, left main coronary artery; LAD, left anterior descending artery; LCx, left circumflex artery; RCA, right coronary artery; true bifurcation (1,1,1; 1,0,1; 0,1,1); RD, reference diameter; MLD, minimal lumen diameter.
Categorical variables are expressed as No. (%) and continuous variables as mean±standard deviation or median [interquartile range].
The procedural data and in-hospital outcomes are shown in table 3. Regarding technical aspects, there were no differences in the percentage of MV predilation, MV stent size and SB balloon size between the 2 groups. POT was more frequently performed in the iFR group, while SB interventions (postdilation/kissing balloon) were less often required in this group (32.3% vs 56.1%, P=.001). SB stenting was also less frequently performed in the iFR group, but the difference was not significant between groups (0.0% vs 2.6%, P=.104). The stepped PS approach of CBLs treated with the jailed pressure wire technique is detailed in . In the pressure wire group, drift was present in 7 cases. After recalibration, rewiring with the same pressure wire was possible in 6 of them. In the remaining case, rewiring was unsuccessful (with the same guidewire, a new pressure wire and dedicated guidewires). The baseline variables used in the IPTW models for microscopic damage and clinical events were well balanced after weighting, with standardized mean differences <10% for all the covariates (). Angiographic success (residual stenosis <30%) was obtained in all of the MVs in both groups. Postprocedural SB diameter stenosis was greater in the iFR group, but final iFR was> 0.89 in all cases. The rates of in-hospital adverse events were very low and did not differ between groups.
Procedural characteristics and in-hospital outcomes
| iFR (n=99) | Control (n=114) | P | |
|---|---|---|---|
| Main vessel | |||
| Predilation | 35 (35.4) | 49 (42.9) | .256 |
| Stent diameter, mm | 2.9±0.4 | 3.0±0.4 | .089 |
| Stent length, mm | 20.0±11.5 | 20.0±10.0 | 1.000 |
| Postdilation (POT) | 73 (73.7) | 46 (40.3) | .001 |
| Side branch | |||
| Predilation | 11 (11.1) | 41 (35.9) | .001 |
| PD (including KB) | 32 (32.3) | 64 (56.1) | .001 |
| Balloon diameter | 2.5±0.5 | 2.4±0.4 | .488 |
| Stenting | 0 (0.0) | 3 (2.6) | .104 |
| Procedural results | |||
| MV stenosis | 5 [2-10] | 4 [2-9] | .191 |
| SB stenosis | 14 [5-40] | 12 [5-24] | .002 |
| In-hospital outcomes | |||
| Post-procedure MI | 1 (1.0) | 2 (1.7) | 1.000 |
| Stroke | 0 (0.0) | 0 (0.0) | 1.000 |
| Major bleeding | 0 (0.0) | 0 (0.0) | 1.000 |
| Death | 0 (0.0) | 0 (0.0) | 1.000 |
iFR, instantaneous wave-free ratio; KB, kissing balloon; MI, myocardial infarction; PD, postdilation; POT, proximal optimization technique.
Categorical variables are expressed as No. (%) and continuous variables as mean±standard deviation or median [interquartile range].
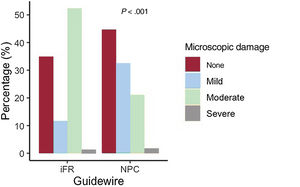
There were no cases of wire fracture in either group. Microscopic analysis was performed in 86 pressure wires and in 114 nonpolymer-coated wires. The primary endpoint of significant microscopic damage was more frequently observed in the pressure wire group than in nonpolymer-coated wire group (53.5% vs 22.8%) (P <.001). This difference remained significant after IPTW adjustment (Padj<.001). The presence of any type of microscopic damage was 65.1% and 55.3% (P=.159), respectively. The degree of microscopic damage in each group is shown in figure 3. Severe damage was observed in 1 pressure wire and in 2 nonpolymer-coated wires (1.2% vs 1.8%, P=.738). In the pressure wire group, drift (n=7) was not associated with the presence of significant microscopic damage (57.1% vs 53.5%, P=.566).
Clinical outcomesAfter a 2-year follow-up, the secondary endpoint of MACE occurred in 15 patients: 3 (3.0%) in the iFR group and 12 (10.5%) in the control group (HR, 0.27; 95%CI, 0.07-0.97; P=.045). The difference did not remain significant in the IPTW-adjusted Cox model (HRadj, 0.42; 95%CI, 0.10-1.73; P=.229). Survival analyses of the unweighted and weighted populations are shown in figure 4. Rates of the individual components of MACE are shown in table 4.
Two-year clinical outcomes according to the use of iFR
| Unadjusted | Adjusted (IPTW) | |||||
|---|---|---|---|---|---|---|
| iFR | Control | HR (95%CI) | P | HR (95%CI) | P | |
| MACE | 3 (3.0) | 12 (10.5) | 0.27 (0.07-0.97) | .045 | 0.42 (0.10-1.73) | .229 |
| Cardiac death | 1 (1.0) | 3 (2.6) | 0.37 (0.04-3.59) | .394 | 0.43 (0.04-4.38) | .485 |
| Myocardial infarction | 1 (1.0) | 3 (2.6) | 0.37 (0.04-3.56) | .390 | 0.75 (0.09-6.54) | .803 |
| Clinically-driven TVR | 1 (1.0) | 6 (5.3) | 0.18 (0.02-1.53) | .118 | 0.16 (0.02-1.29) | .093 |
HR, hazard ratio; iFR, instantaneous wave-free ratio; IPTW, Inverse probability of treatment weighting; MACE, major adverse cardiovascular outcomes; TVR, target vessel revascularization.
Values are expressed as No. (%).
The main findings of this study can be summarized as follows: a) pressure wires showed higher rates of significant microscopic damage than conventional nonpolymer-coated wires; b) the use of the pressure wire as a jailed wire reduced SB interventions after MV stenting; c) iFR can be used to assess the physiological result of the SB and to guide PCI decision making after PS strategy; d) the 2 groups had comparable clinical outcomes after a 2-year follow-up.
Jailed wire technique and microscopic damagePS is the most widely used strategy to treat CBLs and entails MV stent deployment jailing the ostium of the SB. The use of the jailed wire technique, which consists of placing a wire in the SB before MV stenting, is recommended by the European Bifurcation Club.2,15 This maneuver prevents SB occlusion and facilitates reopening if necessary, being especially useful when there are severe stenoses in the MV or SB.16,17 One of the main concerns when using this technique is that wire damage or even fracture may occur during withdrawal.18–20 Several lesion characteristics such as the angle and the presence of calcification or tortuosity have been associated with wire damage. Moreover, some wires are more vulnerable than others.21 In a previous randomized trial, our group described higher rates of damage in nonpolymer-coated wires than in polymer-coated wires.4 In addition, in a pilot study, we observed that 3 out of 4 pressure wires showed some type of microscopic damage when used as jailed wires.10 Thus, in the current study, we aimed to compare microscopic damage in pressure and nonpolymer-coated wires while accounting for lesion characteristics and technical factors that may act as confounders. Using the same classification of microscopic damage as in the above-mentioned randomized trial, we found that the presence of at least moderate microscopic damage was more than twice as high in the pressure wire group. This difference remained significant after IPTW-adjustment for potential confounders: bifurcation location, bifurcation angle, bifurcation type, MV lesion length, MV diameter, SB diameter, severe calcification, severe tortuosity, length of the trapped wire, POT, and SB postdilation. Although POT and other technical factors were well balanced between the groups after the weighing, we cannot completely exclude the possibility that they might have contributed to an increase in the degree of damage. Of note, the damage classification has been adopted from our previous study for academic purpose and reproducibility issues, but structural damage occurs on a continuum. Indeed, when we analyzed any type of damage, the rates did not differ between the 2 groups. In addition, severe damage was only present in 1.2% of pressure wires and there were no cases of wire fracture. In our opinion, these results should be interpreted as a whole and considered along with the lesion characteristics when balancing the pros and the cons of using the jailed pressure wire technique in a specific CBL.
Jailed pressure wire technique to guide coronary bifurcation lesion revascularizationThere is growing interest in the use of coronary physiology to guide PCI decision-making for CBLs. Indeed, this issue has been recently covered in an international position statement by the Korean, Japanese and European Bifurcation Clubs.9 The consensus highlights the usefulness of physiological indexes to determine the functional significance of CBLs and to evaluate the SB result, as well as the need of additional interventions, potentially reducing unnecessary complex procedures.22–27 Accordingly, in our study, the use of an iFR-guided approach was associated with fewer SB angioplasty interventions (postdilation, kissing balloon) and a nonsignificant lower rate of SB stenting. Although these results are consistent with the DKCRUSH VI randomized trial, in which the FFR-guided PCI group underwent fewer SB interventions than the angiography-guided PCI group, it should be noted that due to the nonrandomized design of the present study, this might be explained by anatomical and plaque distribution differences in the CBLs in the 2 groups.6 In addition to the use of distinct physiological indexes, one of the main technical differences between the current study and the above-mentioned trial is that, in that trial, the jailed pressure wire technique was not used. Indeed, SB access and FFR measurement after MV stenting could not be successfully achieved in 15 (9.4%) patients, underlining the difficulty of SB rewiring and the potential usefulness of the jailed pressure wire technique in this setting.11,28 In contrast, one of the limitations of the jailed pressure wire technique is the possibility of drift after MV stenting, which occurred in 7.1% of the procedures and implied recalibration and rewiring of the SB. We believe that drift is related to the pressure wire model (Volcano Verrata and Verrata Plus, Philips, The Netherlands) and not with the jailed technique.
Clinical eventsPhysiology-guided PCI improves clinical outcomes at follow-up.29–31 However, regarding CBLs, no study has demonstrated the superiority of functional-guided vs angiography-guided PCI in terms MACE. In our study, the iFR-based strategy was not superior to the angiography-based strategy in terms of clinical outcomes after weighting for potential confounding factors. Nevertheless, the clinical outcomes propensity score model of the present study did not include anatomical factors such as the presence of a true bifurcation lesion, which should be considered when interpreting our results. Contrary to angiography-guided conservative SB intervention strategies, which have been associated with better clinical outcomes,32 the reduction of SB interventions in the DKCRUSH VI trial and other nonrandomized studies did not translate into a better clinical prognosis at follow-up.6,22,23,33,34 The most plausible explanation for this could be that the functional significance of an SB is not equivalent to its clinical relevance, which is related to the supplying myocardial mass. Based on this consideration, the intervention of a functionally significant but clinically irrelevant SB might be detrimental. Thus, in our opinion, the use of the jailed pressure wire technique should be balanced, accounting not only for unfavorable factors associated with wire damage but also for the potential benefit of using it considering the clinical relevance of the SB.
LimitationsThe main limitations of the study are as follows. First, the design of the study was observational and retrospective. Second, the conformation of the control group with patients from a prior trial from our group that aimed to control for unmeasured and unknown demographic, operator, and procedural related factors, also resulted in slight technical differences between the groups due to temporal changes in standard PCI practices, such as the more frequent use of POT in the iFR group. Although inverse probability weighting with propensity scores resulted in a good balance of the selected covariates, including POT, the presence of residual confounding cannot be completely ruled out. Third, the sample size was calculated for the microscopic damage outcome but might be insufficient to detect differences in clinical outcomes. Finally, although drift assessment was evaluated after MV stent deployment, we cannot completely rule out the possibility of iFR measurement variability during jailing.
CONCLUSIONSPressure wires were less resistant to jailing than conventional nonpolymer-coated wires. However, severe damage was anecdotal and there were no cases of wire fracture, supporting the use of the jailed pressure wire technique in selected patients. The iFR-guided PS strategy resulted in fewer SB interventions, but these patients had similar 2-year clinical outcomes to those who underwent the angiography-guided technique.
FUNDINGThis work was financed within the framework of the Plan Estatal de Investigación Científica y Técnica y de Innovación, with the projects of the Instituto de Salud Carlos III (ISCIII) PI18/00750 and PI12/00440.
AUTHORS’ CONTRIBUTIONSF. Hidalgo and R. González-Manzanares contributed equally to the present work as first authors. F. Hidalgo, R. González-Manzanares, S. Ojeda and M.Pan conceived and designed the study. D. Pastor-Wulf, G. Flores-Vergara, I. Gallo, J. López and G. Dueñas collected and analyzed the data and interpreted the results. F. Hidalgo, R. González-Manzanares, S. Ojeda, and M. Pan drafted the manuscript and completed the critical revisions. J. Suárez de Lezo, M. Romero S. Ojeda and M. Pan reviewed and revised the manuscript and approved its final version before submission. All authors gave their final approval to the version to be published.
CONFLICTS OF INTERESTF. Hidalgo (minor lecture fees: Philips), S. Ojeda (minor lecture fees: Philips, Boston, World Medical, Medtronic, Edwards), M. Pan (minor lecture fees: Philips, Abbott, Boston, World Medical. The other authors have no conflicts of interest to declare.
- -
During PCI, the use of the jailed pressure wire technique may facilitate the physiological approach of coronary bifurcation lesions. However, pressure wires might be particularly vulnerable to retrieval damage.
- -
Significant microscopic damage was more frequent in pressure wires than in nonpolymer-coated wires. The use of the jailed pressure wire technique resulted in fewer SB interventions. MACE rates were similar between the iFR-guided and angiography-guided groups after a 2-year follow-up.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2022.11.004