Lactate and its evolution are associated with the prognosis of patients in shock, although there is little evidence in those assisted with an extracorporeal venoarterial oxygenation membrane (VA-ECMO). Our objective was to evaluate its prognostic value in cardiogenic shock assisted with VA-ECMO.
MethodsStudy of patients with cardiogenic shock treated with VA-ECMO for medical indication between July 2013 and April 2021. Lactate clearance was calculated: [(initial lactate − 6 h lactate) / initial lactate × exact time between both determinations].
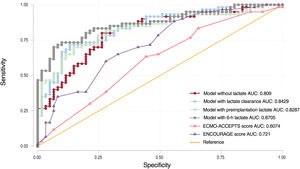
ResultsFrom 121 patients, 44 had acute myocardial infarction (36.4%), 42 implant during cardiopulmonary resuscitation (34.7%), 14 pulmonary embolism (11.6%), 14 arrhythmic storm (11.6%), and 6 fulminant myocarditis (5.0%). After 30 days, 60 patients (49.6%) died, mortality was higher for implant during cardiopulmonary resuscitation than for implant in spontaneous circulation (30 of 42 [71.4%] vs 30 of 79 [38.0%], P=.030). Preimplantation GPT and lactate (both baseline, at 6hours, and clearance) were independently associated with 30-day mortality. The regression models that included lactate clearance had a better predictive capacity for survival than the ENCOURAGE and ECMO-ACCEPTS scores, with the area under the ROC curve being greater in the model with lactate at 6 h.
ConclusionsLactate (at baseline, 6h, and clearance) is an independent predictor of prognosis in patients in cardiogenic shock supported by VA-ECMO, allowing better risk stratification and predictive capacity.
Keywords
Despite the considerable advances in the treatment of patients with ventricular dysfunction and the widespread practice of primary angioplasty, the incidence of cardiogenic shock and related mortality has hardly changed in the last 2 decades.1–4
In recent years, there has been an upsurge in the use of mechanical circulatory support for acute cardiovascular disease, although the efficacy of this measure has not been proven in clinical trials2–5 and the available studies compare heterogeneous groups of patients. Hence, substantial effort is now focused on stratifying the severity of cardiogenic shock to improve selection of circulatory support candidates. For this purpose, it would be of particular value to have laboratory parameters to help guide decision-making.1–6
Blood lactate acid levels provide information on the magnitude of shock and the clinical status of critically ill patients, as there is a proven relationship between elevated lactate values and mortality.7–9 Nonetheless, single determinations have limitations,10,11 and interest has emerged regarding lactate clearance.10,12–19 This parameter also correlates with the prognosis of shock, and its dynamic values provide additional information on the progress of this condition.16,20 However, there is little evidence on its value in cardiogenic shock patients receiving mechanical circulatory support.18,19,21
The primary aim of this study was to analyze the prognostic performance of single lactate acid determinations (before and 6hours after implantation) and lactate clearance in a large sample of patients in cardiogenic shock due to a medical cause and undergoing venoarterial extracorporeal membrane oxygenation (VA-ECMO). The secondary aim was to analyze the added value and predictive capability of lactate (at baseline, 6hours, and clearance) with respect to other factors.
METHODSStudy participantsWe identified all consecutive cardiogenic shock patients treated with VA-ECMO for a medical indication, hospitalized in the cardiac critical care unit of our center between July 2013 and April 2021. Baseline and event data were collected and analyzed retrospectively. Patients lacking arterial blood lactate measurements at admission and at 2hours and 6hours after VA-ECMO implantation, and patients who were cannulated to undergo a high-risk procedure (eg, ventricular tachycardia ablation, complex percutaneous revascularization) were excluded from the analysis.
Indications and treatmentThe indications for VA-ECMO implantation were the following: a) refractory cardiogenic shock, b) pulmonary thromboembolism in shock with a contraindication for fibrinolysis, and c) in-hospital cardiac arrest refractory to advanced cardiopulmonary resuscitation for more than 10minutes. The absolute contraindications included comorbidity that determined a reduced life expectancy, established and irreversible multiorgan failure, and uncontrollable active bleeding.
Once the decision to start VA-ECMO support had been made, informed consent was obtained and the cardiac catheterization team (available 24hours a day) performed ultrasound- and fluoroscopy-guided cannulation using the Seldinger technique. When circumstances made it impossible to transfer the patient to the cardiac catheterization laboratory, ultrasound-assisted cannulation took place at the bedside. A femoro-femoral access was performed with 15-Fr or 17-Fr cannulas for outflow and 21-Fr or 23-Fr for inflow. Whenever possible, the superficial femoral artery was cannulated (5 Fr or 6 Fr) for antegrade perfusion.
After completing VA-ECMO implantation, patients were admitted to the cardiac critical care unit and maintained on invasive mechanical ventilation, sedation, and analgesia. Therapeutic hypothermia was used in patients who were in cardiac arrest during implantation and those in deep shock after arrest, controlling the temperature with the VA-ECMO heater to reach a target of 34C for 24hours in the absence of contraindications. Anticoagulation was carried out by continuous sodium heparin infusion to reach an activated partial thromboplastin time ratio of 1.5-2, together with daily anti-Xa monitoring (target, 0.3-0.5 U/mL).
If organ failure and cardiac and respiratory function had recovered following support therapy, a weaning test was performed with subsequent decannulation when the response was favorable. Decannulation was done by surgery or percutaneous vascular closure using 2 Perclose ProGlide devices (Abbott, United States).22 The second option was preferred for cannulations performed in the cardiac catheterization laboratory with no technical problems at the puncture site.
Definition of lactate clearanceLactate clearance was calculated as the baseline lactate value (the highest before the start of circulatory support) minus the 6-hour value, divided by the product obtained on multiplication of the baseline value by the interval in minutes between the 2 measurements, according to the formula described by Fuernau et al.,20 which is designed to correct the time variation between determinations when it is not prespecified. A negative value reflects an increase in lactate since the start of treatment, whereas a positive value is directly proportional to lactate clearance.
Data collectionThree investigators retrospectively collected information on demographics, clinical and implant-related variables, and therapy outcome from the patients’ electronic medical and nursing records up to April, 2021. The definitions used were those from the Extracorporeal Life Support Organization (ELSO) registry.23
Long-term follow-up was carried out by retrospectively reviewing the electronic medical records available for patients from the same autonomous community as our center. For the remaining (exceptional) cases, patients or their referral centers were contacted by telephone.
ENCOURAGE and ECMO-ACCEPTS scores were calculated as described in the respective original publications.24,25 The prothrombin activity value of <50% required for the ENCOURAGE score was assumed to be equivalent to an international normalized ratio (INR) value >1.5. Each patient was assigned a shock stage using the Society for Cardiovascular Angiography and Intervention (SCAI) classification.1,6
The study was approved by the clinical research ethics committee of our center with the code ECMO19-270/19.
Statistical analysisQuantitative variables are described as the mean±standard deviation or the median [interquartile range] in those with a nonnormal distribution according to the Kolmogorov-Smirnov test. Qualitative variables are described as number and percentage. Variables predictive of mortality at 30 days after the start of VA-ECMO were analyzed using univariate logistic regression. As lactate clearance and lactate values at certain time points can be interdependent, multivariate logistic regression analysis was done to avoid overfitting the model. Four models were configured: without lactate, with lactate clearance, with lactate before VA-ECMO implantation, and with lactate at 6hours postimplantation. In all models, we included variables showing a P value <.20 on univariate analysis, as well as variables from the ENCOURAGE score (except lactate), included separately. Each model underwent a tolerance analysis, which gave low?? variance inflation factor values and confirmed the absence of collinearity between variables. The area under the receiver-operating characteristic (ROC) curve (AUC) of each logistic regression model was compared by the c-statistic to obtain the net reclassification improvement (NRI) and the integrated discrimination improvement (IDI) indexes. These AUCs were compared with those of the ENCOURAGE and ECMO-ACCEPTS scoring systems. The 4 models were simplified to the smallest number of variables possible using a backward stepwise method, and AUCs were also obtained for the reduced predictive models. Based on the Youden index, the cutoff points for preimplantation lactate, 6-hour postimplantation lactate, and lactate clearance providing best differentiation were obtained with a survival analysis using Kaplan-Meier curves. In addition, a multivariate analysis for mortality was carried out with the 3 lactate models (baseline, 6hours, and clearance) using Cox regression, including a follow-up time up to April 2021. Statistical analyses were performed with the SPSS program, 19.0 (IBM, United States)
RESULTSOf the 149 patients treated with VA-ECMO in our center between July 2013 and April 2021, we excluded 28 patients who lacked measurements required to calculate lactate clearance. Most of them had been referred from other centers.
The baseline characteristics of the 121 patients analyzed are shown in table 1. The mean age was 56 years and 77% were men. The ENCOURAGE and ECMO-ACCEPTS scores were recorded to reflect the depth of shock prior to VA-ECMO implantation. The most frequent reasons for this treatment were acute myocardial infarction (AMI) (44, 36.4%), implantation during cardiac arrest (42, 34.7%), high-risk pulmonary thromboembolism (14, 11.6%), arrhythmic storm (14, 11.6%), and fulminant myocarditis (6, 5.0%) (figure 1A). In total, 83 patients (68.6%) simultaneously received intra-aortic balloon pump support for left ventricular unloading, and 12 of them required replacement with an Impella CP (Abiomed, United States) due to ventricular distention with poor pulsatility. A distal perfusion cannula was placed in the superficial femoral artery on the side of the VA-ECMO outflow cannula in 104 patients (85.9%).
Baseline characteristics before establishment of venoarterial extracorporeal membrane oxygenation
| Patients, n | 121 |
| Age, y | 56.3±12.1 |
| Men | 93 (76.9) |
| Hypertension | 51 (42.1) |
| Dyslipidemia | 54 (44.6) |
| Diabetes mellitus | 29 (24.0) |
| Smoking | 67 (55.4) |
| Body mass index | 27.9±4.8 |
| Peripheral vascular disease | 14 (11.6) |
| Previous AF | 17 (14.0) |
| LVEF at implantation, % | 20 [10.0-35.0] |
| SCAI shock stage preimplantation | |
| A, B | 0 |
| C | 5 (4.1) |
| D | 33 (27.3) |
| E | 83 (68.6) |
| Previous ischemic heart disease | 21 (17.4) |
| Intra-arrest implantation | 42 (37.4) |
| Therapeutic hypothermia | 80 (66.1) |
| Glasgow <6 preimplantation | 90 (74.4] |
| Creatinine preimplantation, mg/dL | 1.3 [1.1-1.8] |
| INR preimplantation | 1.3 [1.1-1.6] |
| ALT preimplantation, U/L | 170 [75.0-364.5] |
| Bilirubin preimplantation, mg/dL | 0.9±0.9 |
| ENCOURAGE score | |
| 0-12 | 4 (3.3) |
| 13-18 | 17 (14.0) |
| 19-22 | 22 (18.2) |
| 23-27 | 25 (20.7) |
| ≥ 28 | 53 (43.8) |
| ECMO-ACCEPTS score | 27.0 (23.0-29.0) |
| Lactate preimplantation, mmol/L | 9.4±4.6 |
AF, atrial fibrillation; ALT, alanine aminotransferase; INR, international normalized ratio; LVEF, left ventricular ejection fraction; SCAI: Society for Cardiovascular Angiography and Intervention.
The values are expressed as No. (%), mean±standard deviation, or median [interquartile range].
The main complications occurring in the 121 patients included are shown in figure 1B. At 30 days, 60 (49.6%) patients had died. The main causes of death were multiorgan failure in 22 patients (36.7%), severe anoxic encephalopathy or brain death in 17 (28.3%), intractable bleeding in 5 (8.3%), and sepsis in 5 (8.3%). Mortality was higher in patients undergoing implantation during cardiac arrest than in those with spontaneous circulation (30 of 42 [71.4%] vs 30 of 79 [38.0%]; P=.030). In the univariate analysis, intra-arrest implantation, ENCOURAGE score, ECMO-ACCEPTS score, presence of severe bleeding, SCAI shock stage, preimplantation ALT, preimplantation peak lactate, lactate at 6hours postimplantation, and lactate clearance were associated with prognosis (table 2 and figure 2). The results of the 4 multivariate logistic regression models are shown in table 3. Lactate clearance and the separate lactate determinations were independently associated with 30-day mortality. The results of the multivariate analysis using Cox regression were similar (table 4).
Results of the univariate analysis
| Variables | Mortality at 30 days | OR (95%CI) | P | |
|---|---|---|---|---|
| No (n=61) | Yes (n=60) | |||
| Indication for implantation | ||||
| Myocardial infarction in shock | 26 (42.6) | 18 (30) | 0.58 [0.27-1.22] | .15 |
| Intra-arrest implantation | 12 (19.7) | 30 (50.0) | 4.08 [1.82-9.17] | .001 |
| Arrhythmic storm | 8 (13.1) | 6 (10.0) | 0.74 [0.24-2.27] | .59 |
| Pulmonary thromboembolism | 6 (9.8) | 8 (13.3) | 1.41 [0.46-4.34] | .55 |
| Myocarditis | 5 (8.2) | 1 (1.7) | 0.19 [0.02-1.68] | .14 |
| Tako-tsubo | 1 (1.6) | 0 | — | — |
| Congenital heart disease | 0 | 0 | — | — |
| Post cardiac transplantation | 0 | 0 | — | — |
| Age, y | 54.4±13.4 | 58.1±10.4 | 1.03 [0.99-1.06] | .10 |
| Men | 48 (78.7) | 45 (75.0) | 0.81 [0.35-1.90] | .63 |
| Hypertension | 23 (37.7) | 28 (46.7) | 1.45 [0.70-2.99] | .32 |
| Dyslipidemia | 26 (42.6) | 28 (46.7) | 1.18 [0.58-2.41] | .66 |
| Diabetes mellitus | 17 (27.9) | 12 (20.0) | 0.65 [0.28-1.51] | .31 |
| Smoking | 34 (55.5) | 33 (55.0) | 0.97 [0.47-1.99] | .94 |
| BMI | 27.6±5.3 | 28.2±4.1 | 1.03 [0.96-1.11] | .45 |
| Peripheral vascular disease | 8 (13.1) | 6 (10.0) | 0.74 [0.24-2.27] | .59 |
| Previous AF | 8 (13.1) | 9 (15.0) | 1.17 [0.42-3.27] | .77 |
| LVEF at implantation, % | 15.0 [10.0-30.0] | 20.0 [10.0-43.8] | 1.01 [0.99-1.03] | .16 |
| SCAI shock stage preimplantation | ||||
| A | 0 | 0 | 4.64 [2.02-10.67] | .0001 |
| B | 0 | 0 | ||
| C | 5 (8.2) | 0 | ||
| D | 24 (39.3) | 9 (15.0) | ||
| E | 32 (52.5) | 51 (85.0) | ||
| Previous ischemic heart disease | 13 (21.3) | 8 (13.3) | 0.57 [0.22-1.49] | .25 |
| Glasgow <6 preimplantation | 40 (65.6) | 50 (83.3) | 2.63 [1.11-6.21] | .03 |
| Creatinine preimplantation, mg/dL | 1.3 [0.9-1.5] | 1.5 [1.2-1.9] | 1.52 [0.84-2.75] | .16 |
| INR preimplantation | 1.2 [1.0-1.5] | 1.4 [1.2-1.8] | 1.52 [0.88-2.63] | .14 |
| ALT preimplantation, U/L | 93 [54.5-183.5] | 298.5 [119.0-563.3] | 1.01 [1.00-1.02] | .01 |
| Bilirubin preimplantation, mg/dL | 0.8±0.5 | 1.1±1.2 | 1.44 [0.88-2.35] | .14 |
| Severe lower limb ischemia | 10 (16.4) | 16 (26.2) | 1.86 [0.76-4.50] | .17 |
| Stroke | 1 (1.6) | 5 (8.3) | 5.46 [0.62-48.16] | .13 |
| Severe bleeding | 13 (21.3) | 28 (46.7) | 3.23 [1.46-7.16] | .004 |
| Hemolysis | 0 (0.0) | 1 (1.7) | - | - |
| Sepsis | 14 (23.0) | 7 (11.7) | 0.44 [0.17-1.19] | .11 |
| RRT | 2 (3.3) | 8 (13.3) | 4.54 [0.92-22.34] | .06 |
| ENCOURAGE score | 22.0 [16.5-28.0] | 28.0 [23.0-34.8] | 1.13 [1.06-1.19] | .0001 |
| ECMO-ACCEPTS score | 25.0 [23.0-28.0] | 27.0 [25.0-29.0] | 1.14 [1.00-1.29] | .05 |
| Lactate preimplantation, mmol/L | 7.3±4.0 | 11.5±4.3 | 1.25 [1.14-1.38] | .0001 |
| Lactate 6 h postimplantation, mmol/L | 3.6±2.3 | 8.6±4.7 | 1.49 [1.27-1.74] | .0001 |
| Lactate clearance, %/h | 7.7±4.2 | 4.3±4.6 | 0.84 [0.76-0.92] | .0001 |
ALT, alanine aminotransferase; AF, atrial fibrillation; LVEF, left ventricular ejection fraction; 95%CI, 95% confidence interval; BMI, body mass index; INR, international normalized ratio; OR, odds ratio; SCAI, Society for Cardiovascular Angiography and Intervention; RRT, renal replacement therapy.
Qualitative variables are expressed as the No. (%)of patients who died within 30 days, and quantitative variables as the mean±standard deviation or the median [interquartile range] of patients who died within 30 days.
Multivariate analysis of predictors of 30-day mortality
| Variables | Without lactate | With lactate clearance | With preimplantation lactate | With lactate at 6 h | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | |
| Age, y | 2.26 (0.85-5.84) | .11 | 2.13 (0.77-5.90) | .14 | 2.24 (0.86-5.86) | .10 | 2.25 (0.78-6.55) | .14 |
| Men | 1.31 (0.37-4.69) | .67 | 1.38 0.38-4.93) | .62 | 1.42 (0.39-5.07) | .59 | 1.74 (0.47-6.41) | .40 |
| BMI | 1.52 (0.52-4.51) | .45 | 1.54 (0.49-4.82) | .46 | 1.41 (0.45-4.41) | .55 | 1.25 (0.33-4.68) | .74 |
| SCAI shock stage preimplantation | 2.11 (0.74-5.95) | .16 | 2.18 (0.67-7.03) | .19 | 1.44 (0.47-4.45) | .52 | 1.07 (0.31-3.71) | .92 |
| Intra-arrest implantation | 2.36 (0.82-6.76) | .11 | 2.83 (0.92-8.75) | .07 | 1.98 (0.69-5.72) | .21 | 2.59 (0.81-8.32) | .11 |
| Creatinine preimplantation, mg/dL | 1.08 (0.39-2.98) | .88 | 1.11 (0.48-2.58) | .81 | 1.04 (0.46-2.37) | .92 | 1.06 (0.52-2.15) | .87 |
| INR preimplantation | 1.22 (0.60-2.48) | .58 | 1.34 (0.65-2.75) | .43 | 1.32 (0.64-2.72) | .44 | 1.31 (0.59-2.90) | .50 |
| ALT preimplantation, U/L | 1.01 (1.00-1.02) | .03 | 1.00 (0.99-1.01) | .08 | 1.01 (1.00-1.02) | .03 | 1.01 (1.00-1.02) | .04 |
| Severe lower limb ischemia | 1.74 (0.61-4.95) | .30 | 2.27 (0.78-6.64) | .13 | 1.50 (0.54-4.17) | .44 | 1.81 (0.61-5.35) | .28 |
| Severe bleeding | 2.25 (0.83-6.12) | .11 | 2.18 (0.79-6.02) | .13 | 1.90 (0.71-5.13) | .20 | 1.88 (0.65-5.46) | .24 |
| RRT | 1.60 (0.37-7.06) | .53 | 2.44 (0.49-12.32) | .28 | 1.82 (0.45-7.38) | .41 | 2.85 (0.59-13.68) | .19 |
| Lactate clearance, %/h | 0.84 (0.74-0.96) | .01 | ||||||
| Lactate preimplantation, mmol/L | 1.15 (1.04-1.28) | .01 | ||||||
| Lactate at 6 h, mmol/L | 1.42 (1.21-1.67) | <.01 | ||||||
| Tolerance analysis (collinearity) | VIF 1.24 | VIF1.28 | VIF 1.29 | |||||
95%CI, 95% confidence interval; ALT, alanine aminotransferase; BMI, body mass index; INR, international normalized ratio; OR, odds ratio; RRT, renal replacement therapy; SCAI, Society for Cardiovascular Angiography and Intervention; VIF, variance inflation factor.
Multivariate analysis with Cox regression
| Variables | With lactate clearance | With preimplantation lactate | With lactate at 6 h | |||
|---|---|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | |
| Age, y | 1.51 (0.83-1.90) | .19 | 1.33 (0.81-2.06) | .20 | 1.88 (0.90-2.35) | .16 |
| Men | 1.08 [0.57-2.02) | .82 | 1.13 (0.61-2.08) | .69 | 1.24 (0.70-2.20) | .45 |
| Body mass index | 1.75 (0.87-3.51) | .12 | 1.56 (0.80-3.01) | .19 | 1.24 (0.94-3.88) | .07 |
| SCAI shock stage preimplantation | 2.63 (1.08-6.37) | .03 | 2.01 (0.93-4.38) | .08 | 1.95 (0.80-4.78) | .14 |
| Intra-arrest implantation | 1.92 (0.97-3.81) | .06 | 1.50 (0.78-2.88) | .23 | 1.61 (0.84-3.09) | .16 |
| Creatinine preimplantation, mg/dL | 1.17 (0.83-1.63) | .37 | 1.08 (0.77-1.53) | .64 | 1.08 (0.75-1.56) | .67 |
| INR preimplantation | 1.00 (0.65-1.56) | .98 | 1.12 (0.72-1.72) | .62 | 1.01 (0.61-1.65) | .97 |
| ALT preimplantation, U/L | 1.00 (1.00-1.01) | .02 | 1.00 (1.00-1.01) | <.01 | 1.00 (0.99-1.01) | .28 |
| Severe lower limb ischemia | 0.92 (0.50-1.69) | .78 | 0.84 (0.47-1.51) | .56 | 0.95 (0.53-1.70) | .88 |
| Severe bleeding | 1.50 (0.85-2.66) | .16 | 1.47 (0.85-2.52) | .16 | 1.64 (0.98-2.74) | .06 |
| RRT | 0.58 (0.24-1.40) | .22 | 0.78 (0.41-1.50) | .46 | 0.67 (0.31-1.45) | .31 |
| Lactate clearance, %/h | 0.91 (0.86-0.97) | <.01 | ||||
| Lactate preimplantation, mmol/L | 1.12 (1.05-1.19) | <.01 | ||||
| Lactate 6 h postimplantation, mmol/L | 1.19 (1.12-1.26) | <.01 | ||||
95%CI, 95% confidence interval; ALT, alanine aminotransferase; INR, international normalized ratio; OR, odds ratio; RRT, renal replacement therapy; SCAI, Society for Cardiovascular Angiography and Intervention.
Of note, 30-day mortality was 71.4% in the 7 patients with negative lactate clearance and 90% in the 10 patients with no lactate clearance.
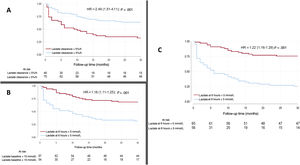
Compared with the model without lactate, the models including lactate clearance, preimplantation lactate, or 6-hour postimplantation lactate showed significantly better prognostic performance: IDI 0.07, 0.04, and 0.14, and NRI, 0.13, 0.12, and 0.18, respectively. The ROC curve analysis for each model is shown in figure 3. The model including 6-hour postimplantation lactate had the highest predictive capacity for 30-day mortality. Comparison of the AUCs with those of the ENCOURAGE and ECMO-ACCEPTS indexes is shown in figure 3. The 4 reduced models, which included only preimplantation ALT, preimplantation indication, and severe bleeding, showed good, but slightly lower AUCs (0.79 without lactate, 0.82 with clearance, 0.81 with preimplantation lactate, and 0.85 with 6-hour lactate). The cutoff points that best dichotomized the sample were 5%/h for lactate clearance, 5 mmol/L for 6-hour postimplantation lactate, and 10 mmol/L for preimplantation lactate. The Kaplan-Meier curves for these cutoffs are shown in figure 4.
There were no statistically significant differences in the development of lower limb ischemic complications associated with the presence or absence of a distal perfusion cannula. Twenty-six patients experienced ischemia (2 of them had no cannula in the superficial femoral artery), whereas 95 patients had no ischemia (15 of them had no cannula).
DISCUSSIONThe main finding of this study is that lactate acid determination and the changes in this parameter are independently associated with the prognosis of patients in cardiogenic shock receiving VA-ECMO support. For every 1% per hour increase in lactate clearance, mortality decreased by 16%. Furthermore, patients with >5% per hour clearance had a 2.5-fold higher probability of survival.
In a situation of general hypoperfusion, lactate production by all organs increases to an amount disproportionate to the degree of clearance achieved by the liver and kidneys,26 which become increasingly more dysfunctional. Thus, lactate accumulates progressively in the blood and is an early marker of tissue hypoxia. Lactate acid elevation actually correlates better with the degree of hypoperfusion than does the mean arterial pressure, which is initially maintained due to sympathetic hyperactivation. Therefore, hypoperfusion is not merely a macrocirculation problem; it is a more complex microvascular and metabolic phenomenon in which a vicious cycle is perpetuated, triggering a more pronounced inflammatory response, endothelial dysfunction, coagulopathy, vasoplegia, and multiple organ failure.11
However, single lactate acid determinations have several limitations. The normality value varies according to the cause of shock, underlying comorbidity, interindividual metabolic variability, and the degree of liver and kidney damage, which can produce lactate elevations that are inconsistent with the existing tissue hypoperfusion.10,11 Hence, in recent years, special interest has focused on the dynamic changes occurring in blood lactate values.10–19,21
Lactate clearance has been investigated mainly in noncardiogenic shock. Persistent lactate elevations have shown a more robust association with mortality than single measurements.12–15 In a study by Nguyen et al.,10 including 111 patients with septic shock, lactate clearance at 6hours was the only variable independently related to mortality.
The available information in acute cardiovascular disease is scarcer and more controversial. Nonetheless, the overall evidence seems to indicate that a persistent lactate elevation confers a poorer prognosis. In this line, 2 studies stand out. In a sample of 51 patients in shock after AMI, Atanná et al.17 found that lactate clearance <10% at 12hours was an independent predictor of early mortality. In a substudy of the IABP-SHOCK II trial, Fuernau et al.20 reported a significant association between 30-day mortality and lactate levels (baseline, 8hours, and clearance). Lactate determination at 8hours showed the greatest predictive capacity, and a value of <3.1 mmol/L was proposed as the best cutoff to identify survivors.
In cardiogenic shock patients receiving VA-ECMO support, 2 studies should be highlighted, one by Slottosch et al.18 and the other by Mungan et al.,19 mainly including postoperative patients. In both series, patients were in deep shock, as indicated by mean lactate levels at implantation of around 10 mmol/L and mortality rates of 60%. Lactate clearance at 12hours postimplantation was found to be a useful predictor of survival in the study by Slottosch18 and clearance at 2hours postimplantation in the study by Mungan.19 Among the remaining variables recorded, only age was independently associated with survival.
A study by Scolari et al.21 described the findings in 43 patients treated with mechanical circulatory support (58% with VA-ECMO). In this case, the patients included were in a less severe condition, with mean lactate at implantation around 6 mmol/L and most indications for a medical cause (44% AMI and 23% heart failure). Therefore, the series was more similar to the patients reported here, although it had a certain percentage of postoperative patients, lower mean lactate value, lower average ENCOURAGE score, and fewer intra-arrest implantations. Mortality at 30 days was 72% and lactate clearance at 6hours was associated with survival.
Lactate acid status also has prognostic value in intra-arrest and postarrest cases. In the study by Masyuk et al.27 including 112 patients with postcardiac arrest shock treated by mechanical circulatory support, baseline lactate >10 mmol/L was associated with >95% mortality. In our setting, a study by Couto et al.28 found that preoperative lactate was a strong predictor of immediate events following heart transplantation in patients treated with circulatory support.
In recent years, efforts have been directed toward better selection of candidates for circulatory support to avoid futile procedures in extreme cases and unnecessary complications in less severe ones. For this purpose, scoring scales have been developed to define prognostic categories based on the results from various cohorts of patients in cardiogenic shock receiving VA-ECMO. The most important examples are the ENCOURAGE (limited to AMI patients), ECMO-ACCEPTS, and SAVE scores, although this last scale does not include intra-arrest implantation and the variables used were initially designed for venovenous ECMO.24,25,29
However, these predictive models only evaluate preimplantation characteristics. In this regard and unlike static models, the recently published VA-ECMO PREDICT30 score is easily applicable and has a dynamic character analogous to lactate clearance. In an external validation cohort, this scoring system showed greater predictive capacity than several others, including SAVE, APACHE, SOFA, and SAPS.
The present study provides the first reported experience in our setting of VA-ECMO care in cardiogenic shock. Unlike the above-mentioned studies, the patients included had shock with a medical cause. In most of the intra-arrest implantations, there was an underlying acute coronary syndrome. It should be noted that VA-ECMO was not indicated for shock after cardiotomy or heart transplantation, or for congenital heart disease, etiologies with different prognoses according to the SAVE score.29
Liver damage was more common in patients who died within 30 days, whereas acute renal failure was not statistically associated with mortality. In similar patients, such as those included in the study by Fuernau et al.,20 organ injury did not show a better correlation with survival than lactate level or lactate clearance, whereas in some studies, such as the one by Scolari et al.,21 lactate was not an independent predictor of prognosis.
The main complications in our cohort were similar to those previously reported in association with VA-ECMO support.2 All cannulations by percutaneous access were done by interventional cardiologists, and the ischemic complication rate was similar to values described in other series.2 The presence of a distal perfusion cannula was not associated with fewer lower limb ischemic complications, although there may have been some selection bias. The mortality results (50%) should be highlighted, considering the patients’ complex clinical status and the severity of shock. The findings in older cohorts, such as the validation of ECMO-ACCEPTS,25 ENCOURAGE,24 VA-ECMO PREDICT,30 and SAVE,29 and the studies by Slottosch et al.,18 Mungan et al.,19 Scolari et al.,21 and Rosselló et al.,31 showed higher mortality.
The logistic regression models including preimplantation lactate, 6-hour lactate, or lactate clearance had greater discriminative power than the model without lactate. The AUC of 6-hour lactate showed the most robust predictive capacity, in keeping with the findings of Scolari et al.21 and Fuernau et al.20 Mortality was dramatically higher in patients with negative or no lactate clearance. This association was maintained for all the diagnostic groups, including patients with intra-arrest implantation a subgroup with high mortality, mainly because of neurological damage.
In light of the reported evidence and our results, serial determination of arterial lactate in patients in cardiogenic shock is important for the following reasons:
- 1.
The initial value is useful for risk stratification and selecting the type of circulatory support based on shock severity (pure cardiogenic or mixed cardiometabolic). Patients with lactate values>5 mmol/L20 may be in more advanced phases (SCAI D or E) and may not respond to left ventricular assistance alone.1,3,6 Thus, in doubtful cases, it seems increasingly advisable to guide decisions by hemodynamic evaluation using a Swan-Ganz catheter.1–5,31
- 2.
In the treatment of cardiogenic shock, a priority is to achieve the highest possible clearance in early phases, as this indicates restoration of adequate tissue perfusion.
- 3.
Prompt identification of patients not achieving adequate clearance allows exclusion of the typical complications occurring in hyperlactacidemia (vascular injury, intestinal ischemia, sepsis, and concomitant bleeding) and consideration of escalating care (Impella implant or surgical ventricular assistance) if there is persistent pulmonary congestion due to ventricular distention with low pulsatility.
The most useful time point for measuring lactate clearance is currently uncertain, and those most widely used are 6hours, 8hours, and 12hours postimplantation. In future studies it could be of value to include additional determinations to compare clearance at different points. During data collection in the present study, there may have been small variations in the precise time or lactate value recorded by the researchers. However, since the start of the VA-ECMO program in our center, lactate recording in the clinical history has been performed systematically at pre-established times. Furthermore, the time difference between the 2 determinations was corrected according to the formula of Fuernau et al.20 Another limitation is the descriptive nature of the study, although this is a common design in the critical care area. Moreover, it was impossible to reliably collect certain parameters from each patient, such as the pH and bicarbonate level, which would have enabled calculation of the VA-ECMO PREDICT score. Finally, it should be noted that the findings did not undergo external validation.
CONCLUSIONSLactate acid status (preimplantation, 6-hours postimplantation, and clearance) is an independent prognostic factor in cardiogenic shock patients treated with VA-ECMO support. These determinations enable better risk stratification and show good predictive performance.
FUNDINGNo funding was received for this study.
AUTHORS’ CONTRIBUTIONSConception and design of the study: J. Martínez-Solano, I. Sousa-Casasnovas, J. García-Carreño. Data collection: J. Martínez-Solano, I. Sousa-Casasnovas, J. García-Carreño, M. Juárez-Fernández, F. Díez-Delhoyo, R. Sanz-Ruiz, C. Devesa-Cordero, J. Elízaga-Corrales. Coordination and presentation to the ethics committee: I. Sousa-Casasnovas, F. Fernández-Avilés, M. Martínez-Sellés. Writing the article, main contributions: J. Martínez-Solano, I. Sousa-Casasnovas, J. García-Carreño, M. Juárez-Fernández, F. Díez-Delhoyo, R. Sanz-Ruiz, C. Devesa-Cordero, J. Elízaga-Corrales, F. Fernández-Avilés, M. Martínez-Sellés. Statistical analysis: J. Martínez-Solano, J.M. Bellón-Cano, M. Martínez-Sellés. F. Fernández-Avilés and M. Martínez-Sellés: similar contributions.
CONFLICTS OF INTERESTNone declared.
- –
Lactate acid determination has shown prognostic value in shock patients. There is now particular interest in the additional benefit provided by the dynamic value of lactate clearance compared with single determinations.
- –
Lactate clearance and lactate level at 6hours are associated with survival in patients in cardiogenic shock receiving VA-ECMO support, a situation for which there is little available information. These determinations can be an early goal of therapy that will enable prompt identification of complications or the need to scale up circulatory support.