No comparisons have been published yet regarding the newest iteration of balloon- and self-expandable transcatheter heart valves for the treatment of bicuspid aortic valve (BAV) stenosis.
MethodsMulticenter registry of consecutive patients with severe BAV stenosis treated with balloon-expandable transcatheter heart valves (Myval and SAPIEN 3 Ultra, S3U) or self-expanding Evolut PRO+(EP+). TriMatch analysis was carried out to minimize the impact of baseline differences. The primary endpoint of the study was 30-day device success, and the secondary endpoints were the composite and individual components of early safety at 30 days.
ResultsA total of 360 patients (age 76.6±7.6 years, 71.9% males) were included: 122 Myval (33.9%), 129 S3U (35.8%), and 109 EP+(30.3%). The mean STS score was 3.6±1.9%. There were no cases of coronary artery occlusion, annulus rupture, aortic dissection, or procedural death. The primary endpoint of device success at 30 days was significantly higher in the Myval group (Myval: 100%; S3U: 87.5%; and EP+: 81.3%), mainly due to higher residual aortic gradients with S3U and greater≥moderate aortic regurgitation (AR) with EP+. No significant differences were found in the unadjusted rate of pacemaker implantation.
ConclusionsIn patients with BAV stenosis deemed unsuitable for surgery, Myval, S3U and EP+showed similar safety but balloon-expandable Myval had better gradients than S3U, and both balloon-expandable devices had lower residual AR than EP+, suggesting that, taking into consideration the patient-specific risks, any of these devices can be selected with optimal outcomes.
Keywords
Bicuspid aortic valve (BAV) is the most common congenital valvular heart disease, occurring in up to 1% of the general population and in 50% of patients requiring aortic valve replacement, with wide regional differences.1 Due to its complex anatomical considerations and the risk of aortic complications, the main randomized controlled trials exploring transcatheter aortic valve implantation (TAVI) have excluded BAV. Although many real-world registries have shown the feasibility of TAVI in BAV patients,2 new challenges have arisen in this population due to the reduction in surgical risk and longer life-expectancy. The growing range of transcatheter heart valves (THVs) aims to limit the risks associated with TAVI and, thus, improve clinical outcomes.
Although preliminary experience has been reported with the new balloon-expandable SAPIEN 3 Ultra (S3U), Myval THV, and the self-expanding (SE) Evolut PRO+(EP+),3–5 to date no study has provided a head-to-head comparison of these 3 THV alternatives in BAV anatomy.
The aim of this multicenter retrospective registry was to compare the early clinical outcomes of the newer iterations of S3U, Myval THV or EP+in a real-world population of consecutive patients with severe symptomatic BAV stenosis.
METHODSStudy design and populationMulticenter retrospective registry performed at 12 institutions. The study protocol was approved by the institutional ethics committees at each participating center and complied with the Declaration of Helsinki. All patients provided written informed consent for the TAVI procedure and inclusion in the registry.
Real-world consecutive symptomatic patients with severe aortic stenosis and BAV morphology were recruited. All patients were treated with the implantation of 1 of the following TAVI devices: the balloon-expandable Myval (Meril Life Sciences Pvt Ltd, India), the balloon-expandable S3U (Edwards Lifesciences, United States) or the EP+(Medtronic, United States). The THV type and size were left to the discretion of the treating physician according to patient's anatomical singularities. In all participating institutions, all devices have been previously used by the main operators and a retrospective analysis of outcomes did not show inter-institutional significant differences in main outcomes.
Study devices and procedureThe Myval THV system, Conformité-Européenne approved since April 2019, is an Indian balloon-expandable TAVI prosthesis6 with a nickel-cobalt alloy frame covered internally and externally in the ventricular portion with polyethylene terephthalate to minimize the potential for paravalvular aortic regurgitation (AR) and 3 leaflets composed of bovine pericardium tissue. It is available in conventional (20, 23, 26, and 29mm), intermediate (21.5, 24.5, and 27.5mm) and extra-large sizes (30.5 and 32mm), all compatible with the 14-Fr sheath. S3U and the EP+have been extensively described previously.7,8 Briefly, the PRO+device is a SE THV with an external porcine pericardial wrap and is available in 23-mm, 26-mm, 29-mm and 34-mm sizes. The Ultra is a BE THV with bovine pericardial leaflets mounted on a cobalt-chromium alloy frame; compared with its predecessor, SAPIEN 3 (Edwards LifeSciences, United States), it adds a textured polyethylene terephthalate outer skirt, which has an approximately 40% increased height that aims to improve annular sealing. It is available in 20-mm, 23-mm, 26-mm, and 29-mm sizes.
Imaging analysisTransthoracic echocardiograms were obtained at baseline and at 30 days of follow-up, and the measured parameters followed the recommendations of the European and the American guidelines.9 The hemodynamic performance of each THV was assessed by the degree of AR, residual transvalvular gradient, estimated aortic valve area (AVA), and severe prosthesis-patient mismatch (defined as an indexed AVA ≤ 0.65cm2/m2 [< 0.55cm2/m2 in obese patients]).10 BAV morphology was determined at baseline echocardiography.
Multidetector computed tomography scans were performed according to the guidelines of the Society of Cardiovascular Computed Tomography.11 The following parameters were calculated: aortic annulus (maximal, minimal, and mean diameters, area, and perimeter), eccentricity index, and degree of calcification (graded moderate-severe if>689 AU). BAV morphology was confirmed by MCT scans and classified according to the Sievers and Schmidtke system.12 The extent of calcification to the outflow tract was defined as any calcium detected 2-mm below the annulus.
Study endpointsThe primary endpoint was 30-day device success, defined by the Valve Academic Research Consortium-3 consensus10 as a composite endpoint including technical success, freedom from mortality, surgery or intervention related to the device or to a major vascular or access-related or cardiac structural complication, and the intended performance of the valve (mean gradient<20mmHg and less than moderate AR). Secondary endpoints were the composite and individual components of early safety at 30 days.10 Other prespecified secondary endpoints were hemodynamic performance at 30-days as assessed by transthoracic echocardiography.
Data collection and statistical analysisAll baseline, procedural and 30-day follow-up data from the study population were retrospectively collected in a dedicated database at each participating institution. Categorical variables are presented as frequencies and percentages, and comparisons between groups were performed using the chi-square test or Fisher's exact test when necessary. Continuous variables are expressed as mean±standard deviation or median [25th-75th interquartile range]. The normal distribution of continuous variables was tested using Kolmogorov-Smirnov test, and graphically tested using the Q-Q plot. Comparisons between groups were performed using the ANOVA test or Kruskal Wallis test according to variable distribution. The Bonferroni post hoc test was used for multiple comparisons. All tests were 2-sided at the .05 significance level.
TriMatch analysis was carried out; propensity scores with 3 separate logistic regression models for each device and 3 distances were calculated between propensity scores for each possible matched triplet using the 3 models. Given those distances, the matched triplets with minimum standardized distance were retained. We established that a participant could be repeated a maximum of 3 times and that all pairs were unique. The variables included in the model are reflected in .
All analyses were performed using R software, version 3.6.1 (R Project for Statistical Computing).
RESULTSClinical, electrocardiographic, and imaging characteristics at baselineBetween January 2018 and January 2022, we included 360 consecutive patients with BAV and symptomatic aortic stenosis who underwent TAVI in the registry (). The global study population was divided into 3 groups depending on the implanted THV: 122 patients (33.9%) Myval THV, 129 patients (35.8%) S3U THV, and 109 patients (30.3%) EP+THV.
The mean age of the study population was 76.6±7.6 years and 101 patients (28.1%) were women. Most patients had low or intermediate surgical risk, with median Society of Thoracic Surgeons Score and EuroSCORE II of 3.6% [1.9-4.9] and 2.6% [1.7-4.2], respectively. A total of 38.3% of the patients had known coronary artery disease and 4.7% had prior coronary artery bypass grafting. Mean left ventricular ejection fraction was 54.7±13.3%, and there were no significant differences according to the THV. Aortic valve area at baseline was lower in the Myval group than in the S3U and EP+groups and the mean aortic gradient (52.3±14.2 mmHg) was also higher (46.7±14.2 mmHg in the S3U group and 48.8±18.4 mmHg in the EP+group, P=.017). Further clinical, echocardiographic, and imaging characteristics are summarized in table 1. Baseline differences were corrected after adjustment (see ).
Main clinical, electrocardiographic, and imaging characteristics at baseline of the global population and according to valve type
| Global population (N=360) | S3U (n=129) | Myval (n=122) | EP+(n=109) | P | |
|---|---|---|---|---|---|
| Baseline characteristics | |||||
| Age, y | 76.6±7.6 | 78.0±6.4 | 73.0±8.2 | 79.0±6.6 | <.001* |
| Female sex | 101 (28.1) | 33 (25.6) | 27 (22.1) | 41 (37.6) | .024* |
| BMI, kg/m2 | 26.1±4.6 | 27.1±4.8 | 25.5±4.9 | 25.6±3.6 | .007* |
| BSA, m2 | 1.8±0.23 | 1.9±0.23 | 1.8±0.23 | 1.8±0.17 | .001* |
| Coronary artery disease | 138 (38.3) | 56 (43.4) | 42 (34.4) | 40 (36.7) | .314 |
| Previous stroke/TIA | 22 (6.1) | 11 (8.5) | 6 (4.9) | 5 (4.6) | .358 |
| Peripheral artery disease | 33 (9.2) | 8 (6.3) | 12 (9.8) | 13 (12.1) | .287 |
| Porcelain aorta | 27 (7) | 10 (7.7) | 10 (8.1) | 5 (4.7) | .675 |
| Chronic kidney disease | 62 (17.2) | 21 (16.1) | 18 (14.8) | 23 (21.1) | .413 |
| Hemodialysis | 4 (1.1) | 3 (2.3) | 0 (0) | 1 (0.9) | .271 |
| Chronic pulmonary disease | 59 (16.6) | 19 (14.7) | 21 (17.5) | 19 (17.8) | .779 |
| Prior heart surgery | 22 (6.8) | 4 (3.1) | 9 (10.3) | 9 (8.3) | .086 |
| Prior CABG | 15 (4.2) | 2 (1.6) | 9 (7.4) | 4 (3.7) | .069 |
| Prior valvular surgery | 9 (2.8) | 1 (0.8) | 4 (4.6) | 4 (3.7) | .194 |
| Prior atrial fibrillation | 78 (21.7) | 38 (29.5) | 19 (15.6) | 21 (19.3) | .022* |
| NYHA functional class III - IV | 195 (54.2) | 79 (61.2) | 78 (63.9) | 38 (34.9) | <.001* |
| STS score, % | 3.6 [1.9–4.9] | 2.7 [1.9–4.1] | 4 [2.1–5.1] | 4 [1.8–5.2] | .163 |
| Electrocardiographic characteristics | |||||
| Sinus rhythm | 280 (77.8) | 96 (74.4) | 101 (82.8) | 83 (73.1) | .249 |
| Atrial fibrillation | 34 (9.4) | 14 (10.9) | 10 (8.2) | 10 (9.2) | .767 |
| Pacemaker | 46 (12.8) | 19 (14.7) | 11 (9) | 16 (14.7) | .310 |
| LBBB | 47 (13.1) | 18 (14.1) | 18 (14.8) | 11 (10.2) | .549 |
| RBBB | 21 (5.9) | 8 (6.3) | 7 (5.7) | 6 (5.8) | .244 |
| First degree AV block | 66 (18.4) | 35 (27.1) | 20 (16.4) | 11 (10.2) | .003* |
| Echocardiographic parameters | |||||
| LVEF, % | 54.7±13.3 | 55.4±13.3 | 53.9±13.5 | 54.7±13.3 | .720 |
| AVA, cm2 | 0.66±0.18 | 0.66±0.16 | 0.63±0.18 | 0.71±0.22 | .007* |
| Mean aortic gradient, mmHg | 49.3±15.7 | 46.7±14.2 | 52.3±14.2 | 48.8±18.4 | .017* |
| Peak aortic gradient, mmHg | 77.2±24.3 | 73.9±21.6 | 81.7±22.6 | 75.5±30.3 | .032* |
| ≥ Moderate AR | 6 (1.6) | 1 (0.8) | 1 (0.8) | 4 (3.7) | .179 |
| CT findings | |||||
| Mean AA diameter, mm | 26.3±3.3 | 27.6±3.1 | 25.3±3.7 | 26.5±3.1 | .144 |
| Eccentricity index | 0.20±0.07 | 0.23±0.07 | 0.19±0.05 | 0.20±0.06 | <.001* |
| AA area, mm2 | 501 [379-587] | 508 [417-572] | 491 [375-567] | 503 [412-589] | .067 |
| Agatston score | 4080 [3013-5691] | 4267 [3007-5752] | 4549 [3673-5623] | 3707 [3059-5104] | .628 |
| LVOT calcification | 56 (15.6) | 20 (15.5) | 16 (13.1) | 20 (18.3) | .106 |
| LM height, mm | 15.2±3.9 | 15.2±4.1 | 16.6±4.4 | 14.8±3.5 | .247 |
| RCA height, mm | 17.2±3.8 | 17.1±3.9 | 19.4±3.1 | 16.6±3.7 | .023* |
| BAV morphology | |||||
| Type 0 | 27 (7.5) | 11 (8.5) | 10 (8.2) | 6 (5.6) | .409 |
| Type 1 | 327 (91.1) | 118 (91.5) | 109 (89.3) | 100 (92.6) | |
| Type 2 | 5 (1.4) | 0 (0) | 3 (2.5) | 2 (1.9) | |
AA, aortic annulus; AR, aortic regurgitation; AV, atrioventricular; AVA, aortic valve area; BAV, bicuspid aortic valve; BMI, body mass index; BSA, body surface area; CABG, coronary artery bypass grafting; CT, computed tomography; EuroSCORE, European System for Cardiac Operative Risk Evaluation; LBBB, left bundle branch block; LM, left main; LVEF, left ventricle ejection fraction; LVOT, left ventricle outflow tract; MR, mitral regurgitation; NYHA, New York Heart Association; RCA, right coronary artery; RBBB, right bundle branch block; STS, Society of Thoracic Surgeons; TIA, transient ischemic attack; TR, tricuspid regurgitation.
Categorical variables are presented as No. (%). Continuous variables are presented as mean±SD or median [IQR].
Median aortic annulus area was 501 [379-587] mm2 with severe calcification of the leaflets in most patients (a median of 4080 UA) and calcification extending toward the left ventricular outflow tract in 15.6% of the patients, with a similar proportion in each device group. Most patients had bicuspid type 1 morphology (91%) but 7.5% had type 0 morphology. There were no differences between the groups.
Procedural and in-hospital outcomesBalloon predilation was more frequently used before Myval than before S3U (71.2% vs 31.8%, P=.001), while postdilation was more often required following EP+(42.9% vs 16.5% with Myval and 13.2% with S3U, P<.001 for both). Prosthesis sizes are summarized in table 2 and in ; intermediate Myval sizes were used in 26.2% of the cases and extra-large sizes in 4.9% of Myval cases and in 32.8% of the EP+cases. The need for a second prosthesis was more frequent in all cases in the EP+group due to THV embolization (4.7% vs 0.8%; P=.033 for EP+vs Myval), but there were no other severe procedural complications such as coronary artery occlusion, annulus rupture, aortic dissection, and procedural death in our study population.
In-hospital and 30-day follow-up outcomes of the matched study population and comparison between different devices
| S3Un=80 | MYVALn=80 | EP+n=80 | P value S3U vs Myval | P value S3U vs EP+ | P value Myval vs EP+ | P value global | |
|---|---|---|---|---|---|---|---|
| In-hospital outcomes | |||||||
| Transfemoral approach | 77 (100) | 77 (100) | 75 (97.4) | .999 | .500 | .500 | .135 |
| Predilation | 34 (44.2) | 54 (70.1) | 61 (79.2) | .003d | <.001d | .296 | <.001d |
| Postdilation | 10 (12.8) | 12 (15.4) | 34 (43.6) | .824 | <.001d | <.001d | <.001d |
| Valve size | |||||||
| S (S3U:20; Myval:20,21.5; EP+:23) | 5 (6.3) | 4 (5.0) | 5 (6.3) | .639 | .307 | .386 | .494 |
| M (S3U:23; Myval:23,24.5; EP+:26) | 8 (10.0) | 22 (27.5) | 20 (25.0) | ||||
| L (S3U:26; Myval:26, 27.5; EP+:29) | 49 (61.3) | 26 (32.5) | 35 (43.8) | ||||
| XL (S3U:29; Myval:29,30.5,32; EP+:34) | 18 (22.5) | 28 (35.0) | 20 (25.0) | ||||
| >1 prosthesis required | 0 (0) | 0 (0) | 0 (0) | - | - | - | - |
| Valve embolization | 0 (0) | 0 (0) | 2 (2.5) | - | .500 | .500 | .135 |
| Coronary artery occlusion | 0 (0) | 0 (0) | 0 (0) | - | - | - | - |
| Annulus rupture | 0 (0) | 0 (0) | 0 (0) | - | - | - | - |
| Aortic dissection | 0 (0) | 0 (0) | 0 (0) | - | - | - | - |
| Conversion to surgery | 0 (0) | 0 (0) | 0 (0) | - | - | - | - |
| Hemodynamic instability | 0 (0) | 0 (0) | 5 (6.3) | - | .063 | .063 | .070 |
| Successful procedure | 80 (100) | 80 (100) | 75 (93.8) | - | .063 | .063 | .070 |
| Procedural death | 0 (0) | 0 (0) | 0 (0) | - | - | - | - |
| Technical successa | 80 (100) | 80 (100) | 75 (93.8) | - | .063 | .063 | .070 |
| 30-day outcomes | |||||||
| PPI | 10 (12.5) | 8 (10) | 15 (18.8) | .791 | .383 | .210 | .261 |
| AKI | 6 (7.5) | 0 (0) | 15 (18.8) | .031d | .035d | <.001d | <.001d |
| All-cause mortality | 2 (2.5) | 0 | 3 (3.8) | .999 | .999 | .999 | .999 |
| Myocardial infarction | 0 (0) | 0 (0) | 0 (0) | - | - | - | - |
| Minor vascular complications | 4 (5.0) | 3 (3.8) | 6 (7.5) | .999 | .727 | .453 | .497 |
| Major vascular complications | 4 (5.0) | 3 (3.8) | 0 (0) | .999 | .125 | .250 | .156 |
| Bleeding>VARC 1 | 5 (6.3) | 0 (0) | 5 (6.3) | .063 | .999 | .063 | .082 |
| Stroke | 6 (7.5) | 1 (1.3) | 3 (3.8) | .125 | .508 | .625 | .150 |
| LVEF, % | 59.63±10.27 | 54.00±9.43 | 57±10.63 | .213 | .193 | .129 | .167 |
| Δ LVEF, % pre/post | 0.19±0.56P=.740e | −2.30±1.40P=.104e | 0.57±1.27P=.651e | .271 | .985 | .297 | .223 |
| AVA, cm2 | 1.45±0.18 | 1.89±0.37 | 1.85±0.46 | <.001d | <.001d | .987 | <.001d |
| Δ AVA, cm2 pre/post | 0.91±0.04P<.001e | 1.22±0.05P<.001e | 1.18±0.07P<.001e | .001d | .001d | .999 | <.001d |
| Mean aortic gradient, mmHg | 12.98±4.36 | 8.55±3.64 | 9.63±5.29 | <.001d | <.001d | .415 | <.001d |
| Δ Mean aortic gradient, mmHg pre/post | −38.5±1.6P<.001e | −44.5±1.6P<.001e | −42±1.7P<.001e | .172 | .630 | .881 | .175 |
| ≥ Moderate AR | 0 (0) | 1 (1.9) | 7 (13.0) | .999 | .016 | .070 | .005 |
| Prosthetic valve thrombosis | 0 (0) | 0 (0) | 0 (0) | - | - | - | - |
| Prosthetic valve endocarditis | 0 (0) | 0 (0) | 0 (0) | - | - | - | - |
| ≥ Moderate prosthetic mismatch | 0 (0) | 0 (0) | 1 (1.3) | - | .999 | .999 | .368 |
| Device successb | 70 (87.5) | 80 (100) | 65 (81.3) | .002d | .405 | <.001d | .001d |
| Early safetyc | 56 (70.0) | 68 (85.0) | 54 (67.5) | .031d | .871 | .022d | .028d |
AR, aortic regurgitation; EP+, Evolut PRO+; PPI, permanent pacemaker implantation; TF, transfemoral; ACI, acute kidney injury; AR, aortic regurgitation; AVA, aortic valve area; LVEF, left ventricle ejection fraction; PPI, permanent pacemaker implantation; S3U, SAPIEN 3 Ultra; VARC, Valve Academic Research Consortium.
Categorical variables are presented as No. (%).
Technical success was measured at the time of leaving the procedure room and described according to VARC-3 criteria as freedom from mortality, successful access, delivery of the device and retrieval of the delivery system, correct positioning of a single THV, and freedom from surgery or intervention related to device.
Device success was described according to VARC-3 criteria as a composite endpoint including technical success, freedom from mortality, surgery or intervention related to the device or to a major vascular or access-related or cardiac structural complication, and the intended performance of the valve (mean gradient<20 mmHg and less than moderate AR).
Early safety was described according to VARC-3 criteria as a composite endpoint including freedom from all-cause mortality, all stroke, VARC type 2 to 4 bleeding, major vascular, access-related or cardiac structural complication, AKI stage 3 to 4, more than moderate AR, new PPI, and surgery or intervention related to the device.
After adjustment, postdilation continued to be more often required following EP+(table 2). There were no differences in valve sizes or in the rate of periprocedural complications. One patient in the EP+group required open surgery due to left ventricular perforation. There was a trend to better outcomes regarding the composite endpoint of technical success with the balloon-expandable devices (100% for both) than with EP+(93.8%; P=.063).
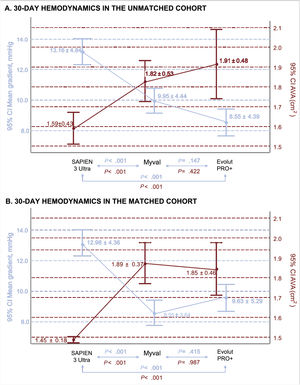
Device success and early safety at 30 days of follow-upThe outcomes of the unmatched cohort are reported in . In the adjusted analysis, the primary composite endpoint of device success at 30 days was significantly higher in the Myval group compared with the S3U group (100% vs 87.5%; P=.002), mainly due to a mean aortic gradient>20mmHg or peak aortic velocity>3m/s (), and also compared with EP+(81.3%; P<.001) due to a higher rate of>moderate AR (figure 1; ). The secondary safety endpoint was also better for Myval than for S3U (85% vs 70%; P=.031), and for EP+(67.5%; P=.022). Pacemaker implantation was required in 46 patients (12.8%) with no significant differences between devices (10.9%, 9.9%, and 18.3% for S3U, Myval and EP+, respectively).
Primary and secondary endpoints at 30-days of follow-up in the matched population.
Device success was described according to VARC-3 criteria as a composite endpoint including technical success, freedom from mortality, surgery or intervention related to the device or to a major vascular or access-related or cardiac structural complication and the intended performance of the valve (mean gradient<20mmHg and less than moderate AR).
Early safety was described according to VARC-3 criteria as a composite endpoint including freedom from all-cause mortality, all stroke, VARC type 2 to 4 bleeding, major vascular, access-related or cardiac structural complication, AKI stage 3 to 4, more than moderate AR, new PPI, and surgery or intervention related to the device.
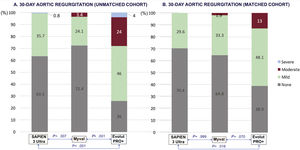
In the matched analysis, predischarge echocardiography showed better hemodynamic performance of Myval vs S3U in terms of aortic valve area (1.89±0.37 cm2 vs 1.45±0.18 cm2; P<.001) and mean aortic gradient (9.9±4.4 mmHg vs 13.1±4.8 mmHg; P<.001). Both parameters were comparable between Myval and EP+(figure 2). However, the rate of moderate or more residual AR was significantly higher for EP+(13%) than for any of the balloon-expandable devices (Myval: 1.9%, S3U: 0%; P=.005 (figure 3). Other prespecified secondary endpoints are listed in table 2.
The main findings of our study are as follows: a) the safety profile of TAVI with new-generation balloon-expandable and SE devices was excellent in patients with BAV stenosis, with no cases of coronary artery occlusion, annulus rupture, aortic dissection, or procedural death, and better global outcomes than those recently reported with previous iterations of balloon-expandable and SE devices; b) after adjustment for the main baseline clinical and anatomical differences, the composite endpoints of device success and early safety were better with Myval than with S3U or EP+but through different mechanisms (better residual gradient than S3U and lower rate of ≥ moderate AR for EP+) which, despite the anticipated selection bias when using newer technologies, suggests the potential benefits of intermediate and extra-large sizes; c) the rate of new pacemaker implantation was low and there were no significant differences among the 3 devices included in this study, likely reflecting the changes in implantation techniques in the last decade.
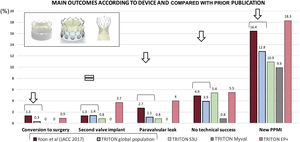
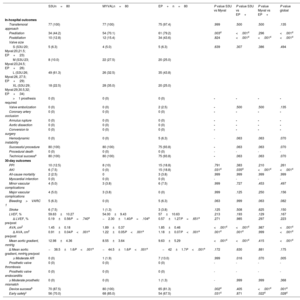
The most widely used devices for the treatment of BAV have been the SE CoreValve/Evolut and the balloon-expandable SAPIEN XT/3 platforms. The first- and second-generation devices of these 2 TAVI platforms have been largely compared, but a head-to-head comparison between the 2 latest iterations is still lacking in BAV. In the landmark study by Yoon et al.,12 main outcomes significantly improved between the 2 former iterations of these devices; according to our findings and as summarized in figure 4, the newest versions of these platforms offer improvements in almost all specific outcomes, but BE devices might outperform SE devices.
Central illustration. Main outcomes in the global study population, according to the device used, and compared with the data reported with former device iterations. Figure elaborated with data from Yoon SH, et al.12. EP+, Evolut PRO+; PPMI, permanent pacemaker implantation; S3U, SAPIEN 3 Ultra.
According to recent European guidelines for the management of valvular heart disease, TAVI is the recommended treatment option for a wide range of patients with symptomatic trileaflet aortic valve stenosis. Conversely, there is no specific recommendation regarding TAVI for patients with BAV stenosis, in whom the default strategy remains surgical aortic valve replacement. The main reason for this is the widely heterogeneous anatomies of this valvulo-arterial disorder: the asymmetric distribution of calcification, the elliptical aortic annulus, the atypical location of coronary artery ostia, the variable phenotypes, and the frequent association with aortic dilation. Indeed, pivotal trials have traditionally excluded patients with BAV stenosis and there is no ongoing trial comparing TAVI to surgical aortic valve replacement.13
Nevertheless, recent studies have reported more favorable outcomes with TAVI than with surgical aortic valve replacement in patients with BAV stenosis, with reduced in-hospital mortality (0.7% vs 1.8%; OR, 0.35; 95%CI, 0.13-0.93; P=.035) and a similar rate of major adverse cardiovascular events at 30 days and 6 months, although TAVI was associated with higher odds of postprocedure need for permanent pacemaker at 30 days.2 Other studies support the feasibility of TAVI in BAV vs trileaflet aortic valve with both balloon-expandable14 and SE15 THV. More recently, the Low-risk bicuspid study16 and the Low-risk TAVI trial17 have reported good clinical outcomes of TAVI in patients with BAV and low surgical risk. All these promising results have allowed an exponential increase in the range of aortic stenosis patients that can be treated with TAVI devices.
Selection of TAVI device in patients with bicuspid aortic stenosisAside from the recent iterations of the most commonly used balloon-expandable and SE THV, the second-generation balloon-expandable Myval THV recently became commercially available with favorable initial clinical outcomes in patients with trileaflet aortic valve stenosis regardless of their surgical risk.3 Moreover, the MATCHBALL study has shown excellent early hemodynamic performance compared with the balloon-expandable SAPIEN 3 THV,16 and Elkoumy et al.5 has recently reported the first case series of Myval THV in BAV stenosis. According to Delgado-Arana et al.,13 intermediate sizes are chosen by ∼40% of operators, addressing the unmet need for a more calibrated THV choice; these additional sizes were only used in around one fourth of our study population but potentially led to a significantly lower residual gradient than S3U and a lower rate of residual AR than EP+, which had an impact on 30-day device success, as defined by Valve Academic Research Consortium-3, but could also affect the long-term durability of this therapy.
Another major concern after TAVI is the need for permanent pacemaker implantation, with known differences between balloon-expandable and SE-THV in trileaflet aortic valve stenosis. The cusp overlap technique is not recommended in patients with BAV, but the lower rate of pacemaker need in patients with SE THV in our cohort compared with historical reports (24.2%-18.3%) suggests that higher implants might also be performed in patients with BAV and that this could minimize the differences with balloon-expandable devices.18
Yoon et al.12 reported more procedural complications with TAVI in BAV than in trileaflet aortic valve stenosis (14.7% vs 8.6%; P=.002) with early-generation devices; these differences were not statistically significant when new-generation devices were used and, according to our finding, this can also be inferred for the new Myval THV (figure 4). Another consideration is that, although previous research suggests similar outcomes in patients with BAV morphology, some potential differences in predictive parameters for paravalvular AR, such as intercommissural distance, merit further investigation.17,18 A definitive prospective randomized clinical trial comparing second-generation THVs is needed to confirm our results and establish the decisive paradigm shift in the treatment of BAV stenosis.
LimitationsThe main limitations of our study are the lack of randomization, its retrospective nature and the absence of data-gathering monitoring, including self-reported echocardiographic outcomes. The learning curve might have negatively impacted the results of patients treated earlier. However, our research started in 2018 and all centers had been performing TAVI for several years prior to the investigation. The lack of long-term follow-up precluded conclusive analysis of cardiovascular mortality or other major events, and consequently the results should be considered hypothesis-generating only. Selection bias may be present since patients were deemed candidates for TAVI through heart team consensus despite their relatively young age and intermediate risk according to STS score, which suggests the presence of comorbidities and anatomical aspects poorly reflected in conventional scores. No differences were detected across centers in terms of global outcomes and with the specific devices. All the centers used balloon-expandable and SE technologies in their clinical practice.
CONCLUSIONSIn this first real-world comparison of the newest generation of balloon- and self-expandable THVs in patients with BAV stenosis deemed not suitable for surgery, the devices showed an excellent safety profile, with better rates of residual regurgitation with both balloon-expandable devices and better residual gradients with self-expandable and Myval devices. The pacemaker implantation rate was similar irrespective of the device. Global outcomes were favorable compared with those reported with prior iterations. These results suggest that, taking into consideration the patient-specific risks, one or other device can be chosen with optimal outcomes.
- -
Bicuspid aortic stenosis is the underlying lesion in a growing proportion of patients deemed candidates for TAVI.
- -
There is little evidence on newer iterations of current TAVI devices.
- -
The latest iteration of balloon-expandable- and SE-THV showed a favorable safety profile, with no coronary artery occlusion, annulus rupture, aortic dissection, or procedural mortality, which represents an improvement compared with global outcomes reported with former iterations.
- -
Device success at 30 days was higher with Myval, mainly due to higher residual gradients with S3U and greater residual regurgitation with EP+. However, the rate of pacemaker implantations showed no significant differences among the 3 devices, suggesting that, taking into consideration the patient-specific risks, one or other device can be chosen with optimal outcomes.
None to declare
AUTHORS’ CONTRIBUTIONSI.J. Amat-Santos and M. García-Gómez should be considered as first authors as they designed the study and undertook the data collection, analysis, interpretation, and writing. F. De Marco, K. Won-Keun, J. Brito, J. Halim, J. Jose, G. Sengotuvelu, A. Seth, C. Terkelsen, M. Protasiewicz, N. Bonilla, B. García, J.P. Sánchez-Luna, S. Blasco-turrión, J.C. González, E. González-Bartol, A.J.J. Ijsselmuiden, and A. San Román performed data collection, analysis interpretation and discussion. I. Gómez-Salvador and M. Carrasco Moraleja performed the data analysis. All authors approved the final version.
CONFLICTS OF INTERESTSNo private funding was received. I.J. Amat-Santos is proctor for Medtronic, Meril Life, and Boston Scientific. B. García has been a proctor for Edwards Lifesciences. Hospital Clinico de Valladolid is receiving unconditional funding for the LANDMARK trial from Meril Life and for the EXPAND-II trial from Medtronic.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2023.03.002