Elderly patients with atrial fibrillation are at greater risk of both cardioembolic events and major bleeding than younger patients. Left atrial appendage occlusion (LAAO) could be an attractive alternative for these patients, but there are limited data on outcomes with LAAO in patients ≥ 85 years old. The aim of the present study was to assess the safety and efficacy of LAAO in patients ≥ 85 years old.
MethodsA total of 1025 patients included in the EWOLUTION registry who underwent LAAO were analyzed and 84 patients ≥ 85 years old were identified and compared with the younger cohort.
ResultsPatients ≥ 85 years old had higher estimated stroke and hemorrhagic risks than younger patients (CHA2DS2-VASc: 5.2±1.2 vs 4.4±1.6, P <.0001; HAS-BLED: 2.7±1.1 vs 2.3±1.2; P=.003; ≥ 85 years vs <85 years). Procedural success was high and similar in both groups (98.8% vs 98.5%; P=.99). There were no differences in 7-day device- or procedure-related adverse event rates (2.6% in ≥ 85 years vs 3.1% in <85 years; P=.80). Despite the higher baseline stroke risk, there was no difference at follow-up between the groups in the annualized stroke rate (0.8/100 patient-years in ≥ 85 years vs 1.3/100 patient-years in <85 years; P=.649).
ConclusionsLAAO in patients ≥ 85 years is safe and effective even though these patients are at high risk for embolic and hemorrhagic events. LAAO may be a reasonable alternative to oral anticoagulation in these patients.
Keywords
Left atrial appendage occlusion (LAAO) has emerged as a nonpharmacologic alternative for stroke prevention in high-risk patients with nonvalvular atrial fibrillation who are deemed to be less than ideal candidates for long-term oral anticoagulation therapy. Several multicenter randomized studies found the treatment to be safe, effective, and noninferior to vitamin K antagonists for stroke prevention,1–3 and longer-term follow-up suggests that cardiovascular mortality may be lower with LAAO.4 As a result, current international guidelines and consensus documents recommend consideration of LAAO in high-risk patients with nonvalvular atrial fibrillation who are either contraindicated or unsuitable for long-term oral anticoagulation, at high bleeding risk, or otherwise prefer an alternative.5,6
There are few data on outcomes with LAAO in elderly patients. In this group of patients, stroke prevention represents an important challenge as aged patients are at greater risk of both cardioembolic events and major bleeding. Elderly patients are potentially ideal candidates for LAAO as this would allow oral anticoagulation discontinuation while maintaining cardioembolic protection. However, because these patients are generally more frail and therefore more prone to complications during interventional procedures, they have been underrepresented in most trials and registries. This analysis compared and contrasted outcomes with LAAO in patients ≥ 85 years old vs younger patients.
METHODSThe present study is a subanalysis of the EWOLUTION registry. The study adhered to international rules for scientific studies, the Helsinki principles, with local ethics committee approval in all participating centers. All patients provided informed consent prior to the procedure. Funding for the study was provided by Boston Scientific Corporation. The design and initial and annual results of the EWOLUTION registry have been published previously.7,8
EWOLUTION was designed as a multicenter, prospective, nonrandomized cohort study. Patients were recruited at each participating center per physician's discretion if they were eligible to receive the Watchman device according to the appropriate local and international guidelines, and were of legal age to provide informed consent. Follow-up for patients was based on the standard practice at each institution, generally a clinical visit between 1 and 3 months postprocedure and yearly thereafter, left atrial appendage imaging to assess residual flow around the device, and annual follow-up visits. The relatedness of each adverse event to the device/procedure was assessed by the participating center. Events and relevant source documents were additionally reviewed by the Sponsor Medical Safety Group.
The objective of the study was to obtain data on procedural success and complications, and long-term patient outcomes, including bleeding and incidence of stroke/transient ischemic attack (TIA)/systemic embolism (SE). The definitions and reporting requirements for adverse event and serious adverse event (SAE) are based on ISO 14155 and the MEDDEV 2.7/3 12/2010. Bleeding was scored according to the Bleeding Academic Research Consortium criteria9; the definition of major bleeding (which includes fatal and life-threatening bleeding) aligns with the LAAO-specific modifications and refinements described by Tzikas et al.10 in the consensus document on definitions, endpoints, and data collection requirements. Stroke was classified in accordance with the Valve Academic Research Consortium criteria.11
All centers were monitored by an outside contract research organization on an ongoing basis and all centers were visited between 1 and 5 times depending on the number of patients enrolled and compliance review to ensure accuracy and completeness of the present follow-up data. All events and relevant source documents were additionally reviewed by the Sponsor Medical Safety Group.
For the purpose of our study, the total cohort was divided into 2 groups (< 85 years vs ≥ 85 years). This arbitrary cutoff age was chosen to select a high-risk group of patients, even though the sample size would be limited. Continuous variables are expressed as mean±standard deviation. Categorical variables are expressed as count and percentage. Baseline characteristics between groups were compared using ANOVA for continuous variables and the Monte Carlo approximation of the Fisher exact test for categorical variables. Rates of events were calculated via the Kaplan-Meier method to account for censoring. P-values were based on log-rank tests for time-to-event analysis. Statistical analysis was performed using the SAS version 9.4 (SAS Institute Inc, Cary, North Carolina, United States).
RESULTSA total of 1025 patients were scheduled for implant in the study in a total of 47 centers in 13 countries. Baseline and acute implant data are, respectively, available for 1025 and 1020 patients, as 5 patients withdrew from the study after giving informed consent with no attempt at, as reported before.8
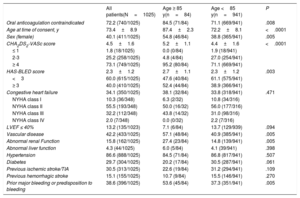
In the total cohort, there were 84 patients ≥ 85 years old. Baseline demographics and risk factors stratified according to age group are summarized in Table 1. Compared with younger patients, patients aged ≥ 85 years had a higher stroke risk (CHA2DS2-VASc: 5.2±1.2 vs 4.4±1.6; P <.0001) and a higher hemorrhagic risk (HAS-BLED: 2.7±1.1 vs 2.3±1.2; P=.003). Elderly patients were also significantly more likely to be women, more likely to have a history of vascular disease or renal dysfunction, more likely to have experienced prior major bleeding or to have a predisposition to bleeding and as a result, were more likely to be considered to have a contraindication to oral anticoagulation.
Baseline characteristics
| All patients(N=1025) | Age ≥ 85 y(n=84) | Age <85 y(n=941) | P | |
|---|---|---|---|---|
| Oral anticoagulation contraindicated | 72.2 (740/1025) | 84.5 (71/84) | 71.1 (669/941) | .008 |
| Age at time of consent, y | 73.4±8.9 | 87.4±2.3 | 72.2±8.1 | <.0001 |
| Sex (female) | 40.1 (411/1025) | 54.8 (46/84) | 38.8 (365/941) | .005 |
| CHA2DS2-VASc score | 4.5±1.6 | 5.2±1.1 | 4.4±1.6 | <.0001 |
| ≤ 1 | 1.8 (18/1025) | 0.0 (0/84) | 1.9 (18/941) | |
| 2-3 | 25.2 (258/1025) | 4.8 (4/84) | 27.0 (254/941) | |
| ≥ 4 | 73.1 (749/1025) | 95.2 (80/84) | 71.1 (669/941) | |
| HAS-BLED score | 2.3±1.2 | 2.7±1.1 | 2.3±1.2 | .003 |
| <3 | 60.0 (615/1025) | 47.6 (40/84) | 61.1 (575/941) | |
| ≥ 3 | 40.0 (410/1025) | 52.4 (44/84) | 38.9 (366/941) | |
| Congestive heart failure | 34.1 (350/1025) | 38.1 (32/84) | 33.8 (318/941) | .471 |
| NYHA class I | 10.3 (36/348) | 6.3 (2/32) | 10.8 (34/316) | |
| NYHA class II | 55.5 (193/348) | 50.0 (16/32) | 56.0 (177/316) | |
| NYHA class III | 32.2 (112/348) | 43.8 (14/32) | 31.0 (98/316) | |
| NYHA class IV | 2.0 (7/348) | 0.0 (0/32) | 2.2 (7/316) | |
| LVEF ≤ 40% | 13.2 (135/1023) | 7.1 (6/84) | 13.7 (129/939) | .094 |
| Vascular disease | 42.2 (433/1025) | 57.1 (48/84) | 40.9 (385/941) | .005 |
| Abnormal renal Function | 15.8 (162/1025) | 27.4 (23/84) | 14.8 (139/941) | .005 |
| Abnormal liver function | 4.3 (44/1025) | 6.0 (5/84) | 4.1 (39/941) | .398 |
| Hypertension | 86.6 (888/1025) | 84.5 (71/84) | 86.8 (817/941) | .507 |
| Diabetes | 29.7 (304/1025) | 20.2 (17/84) | 30.5 (287/941) | .061 |
| Previous ischemic stroke/TIA | 30.5 (313/1025) | 22.6 (19/84) | 31.2 (294/941) | .109 |
| Previous hemorrhagic stroke | 15.1 (155/1025) | 10.7 (9/84) | 15.5 (146/941) | .270 |
| Prior major bleeding or predisposition to bleeding | 38.6 (396/1025) | 53.6 (45/84) | 37.3 (351/941) | .005 |
LVEF, left ventricle ejection fraction; NYHA, New York Heart Association; TIA: transient ischemic attack.
The data are presented as mean±standard deviation or percentage (proportion).
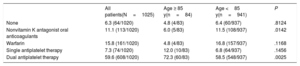
A total of 1020 patients underwent implant attempts and the device was successfully deployed in 1005 of 1020 patients (98.5%). The reasons for the unsuccessful implant attempts were an unsuitable left atrial appendage anatomy (5/15, 0.5% of implant attempts) or inability to meet all device release criteria (9/15, 0.9% of implant attempts). One case was interrupted due to a pericardial effusion likely caused by the pigtail catheter. There were no differences in implant procedure success between the groups (98.8% vs 98.5%; P=0.99 for patients ≥ 85 vs <85 years). Complete seal or jet size ≤ 5mm was achieved in 100% of the patients ≥ 85 years and 99.8% of the patients <85 years, without differences between groups (P=0.99). Postimplantation antithrombotic therapy is summarized in Table 2
Postimplantation antithrombotic therapy
| All patients(N=1025) | Age ≥ 85 y(n=84) | Age <85 y(n=941) | P | |
|---|---|---|---|---|
| None | 6.3 (64/1020) | 4.8 (4/83) | 6.4 (60/937) | .8124 |
| Nonvitamin K antagonist oral anticoagulants | 11.1 (113/1020) | 6.0 (5/83) | 11.5 (108/937) | .0142 |
| Warfarin | 15.8 (161/1020) | 4.8 (4/83) | 16.8 (157/937) | .1168 |
| Single antiplatelet therapy | 7.3 (74/1020) | 12.0 (10/83) | 6.8 (64/937) | .1456 |
| Dual antiplatelet therapy | 59.6 (608/1020) | 72.3 (60/83) | 58.5 (548/937) | .0025 |
There were no differences in the rate of 7-day procedure- and/or device-related SAEs (2.6% in ≥ 85 years vs 3.1% in <85 years; P=.798). The distribution of the periprocedural events in the 2 age groups is shown in Table 3. There was no stroke, death, tamponade, or device embolization in the group of patients ≥ 85 years old.
Major cardiac adverse events within 7 days of implant and other device/procedure-related serious adverse events
| All patientsN=18 (1.8%) | Age ≥ 85 yn=2 (2.4%) | Age <85 yn=16 (1.7%) | |
|---|---|---|---|
| Major adverse cardiac events ≤ 7 d | |||
| All deaths* | 4 | None | 4 |
| Major bleeding | 9 | 2 | 7 |
| Cardiac tamponade/significant PE | 3 | None | 3 |
| Device embolization requiring surgery | 1 | None | 1 |
| Device embolization snared | 1 | None | 1 |
| Stroke | None | None | None |
| Systemic embolism | None | None | None |
| Myocardial Infarction | None | None | None |
| Other events requiring surgery/major intervention | None | None | None |
| N=15 (1.5%) | n=1 (1.2%) | n=14 (1.5%) | |
|---|---|---|---|
| Other periprocedural SAEs ≤ 7 d | |||
| Vascular complications at groin | 4 | None | 4 |
| Air embolism (coronary) | 2 | None | 2 |
| Minor PE (untreated) | 2 | None | 2 |
| Reinterventions due to incomplete seal | 2 | None | 2 |
| Minor bleeding (untreated)/hematoma | 2 | None | 2 |
| TIA | 1 | 1 | None |
| Hypotension | 1 | None | 1 |
| Adverse reaction to anesthesia | 1 | None | 1 |
PE, pericardial effusion; SAE, serious adverse events; TIA, transient ischemic attack.
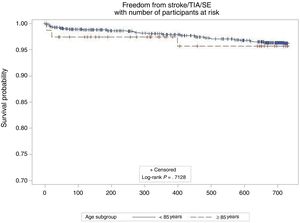
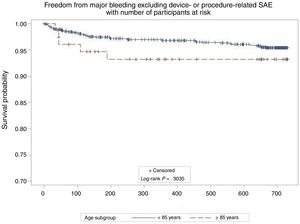
At follow-up (median 732 days; interquartile range, 677-757 days) there were no differences in the annual rates of stroke (0.8/100 patient/y in ≥ 85 years vs 1.3/100 patient/y in <85 years; P=0.649) or stroke/TIA/SE between groups (2.5/100 patient/y in ≥ 85 years vs 2.0/100 patient/y in <85 years; P=.712) (Figure 1). This represented an 80% relative risk reduction compared with the calculated stroke rate in the absence of stroke preventive therapy for similar CHA2DS2-VASc scores12 in both groups (12.2 expected vs 2.5 observed in ≥ 85 years; 9.9 expected vs 2.0 observed in <85 years). There were 3 hemorrhagic strokes in the younger cohort, but none in the elderly cohort. There were no differences in the nonprocedural major bleeding rates (5.1/100 patient/y in ≥ 85 years vs 2.6/100 patient/y in <85 years; P=.286) (Figure 2). These represented 12% and 48% relative risk reductions compared with the calculated bleeding rate for similar HAS-BLED scores in patients ≥ 85 years and <85 years, respectively. At follow-up, 22 patients (29.6%) died in the elderly group and 135 (15.3%) in the in <85 years group. Follow-up transesophageal echocardiogram was available in 79% of the patients in the elderly group and in 88% of the patients <85 years. There were no differences in the rate of device thrombosis (3.9% in ≥ 85 years vs 4.3% in <85 years; P=.8755)
The current study demonstrates that LAAO is a safe and effective procedure in elderly patients (aged ≥ 85 years). The main findings of the present study are as follows: LAAO procedural success rate is comparable in patients aged ≥ 85 years and <85 years; the rate of procedure- and/or device-related SAEs during the procedure or within the first 7 days is also comparable between groups; despite the higher baseline stroke risk in the elderly patients, they achieved a comparable reduction in stroke/TIA/SE with LAAO compared with younger patients.
Elderly patients have a greater risk of cardioembolic events and major bleeding and therefore LAAO could be an attractive alternative for these patients. However, due to their comorbidities, this group of patients has not been represented in most of the trials and registries. Mean age was 75±8.5 years in the AMPLATZER Amulet registry,13 75±8 years in the AMPLATZER Cardiac Plug registry,14 71.8±8.8 years in PROTECT AF,1 and 74.0±7.4 years in the PREVAIL trial.2
To the best of our knowledge only Freixa et al.15 have explored the safety and efficacy of LAAO in elderly patients (< 75 vs ≥ 75 years); they showed that LAAO was associated with similar procedural success in patients aged <75 and ≥ 75 years, and that stroke and major bleeding rates were similar among groups during follow-up. Similarly, the present study has shown a high procedural success in elderly patients (100% in ≥ 85 years) with no differences among younger and older patients. In addition, LAAO appears to be safe in elderly patients with neither procedural stroke nor death in the group of patients ≥ 85 years old. Furthermore, there were no differences in the rate of procedure and/or device-related SAEs during procedure or within the first 7 days between groups.
The average CHA2DS2-VASc score of 5.2±1.2 in patients ≥ 85 years indicates a very high risk of thromboembolic events, corresponding to an expected calculated annual rate of stroke/TIA/SE of 12.2/100 patient/y12 in the absence of stroke preventive therapy. However, the annual stroke/TIA/SE rate observed in this group was only 2.5/100 patient/y, yielding a relative risk reduction of 80%. Although the CHA2DS2-VASc score was lower in the group of patients <85 years (4.4±1.6, P <.001), there were no differences in the annual rates of stroke/TIA/SE between the groups (2.5 vs 2.0; ≥ 85 and <85 years respectively; P=.712).
This analysis suggests that LAAO is safe and effective in elderly patients. On many occasions, elderly patients are considered to be ineligible for oral anticoagulation, but they are often not considered for LAAO due to their higher comorbidity profile and higher expected mortality than those in younger patients, and as a result they are left unprotected against stroke. In the present study, the group of patients ≥ 85 years had more comorbidities than younger patients. However, these differences did not translate into differences in clinical outcomes. The group of very elderly patients had similar success and complication rates during the procedure and throughout 1 year follow-up, there were no differences in the rate of stroke/TIA/SE.
This subanalysis of the EWOLUTION study now provides a larger body of data on outcomes with LAAO in a subgroup of patients in need of an alternative treatment to long-term oral anticoagulation. It has been shown in a real-world setting that, despite achieving better anticoagulation control on warfarin treatment, elderly patients had a higher risk of major hemorrhagic events.16
In addition, elderly patients in clinical trials, as in the studies on direct oral anticoagulants, are generally relatively healthy and adhere to medication; conversely, discontinuation of and nonadherence to direct oral anticoagulants in the older populations are commonly reported in real-world studies.17,18
Study limitationsThis is a subgroup analysis of the EWOLUTION prospective registry with a limited sample size. Limitations of this analysis include the observational nature of the design. The postprocedural antithrombotic regimen was not uniform but at the physicians’ discretion.
CONCLUSIONThis analysis suggests that LAAO is a safe and effective procedure in elderly patients (≥ 85 years). No differences in procedural success or complications were observed in these patients compared with younger patients (< 85 vs ≥ 85 years) and at the 2-year follow-up there were no observed differences in the stroke/TIA/SE rate, despite the high risk of embolic events in very elderly patients. LAAO may be an attractive option in elderly patients with high-risk nonvalvular atrial fibrillation, given the challenges and risks of oral anticoagulation in this growing population.
FUNDINGThe EWOLUTION study was funded by Boston Scientific Inc, Minneapolis, United States.
CONFLICTS OF INTERESTI. Cruz-Gonzalez is proctor and consultant for Boston Scientific and for Abbott Vascular; HI is proctor for Watchman and Lotus (BSC) and has received personal fees from Boston Scientific, outside the submitted work; B. Schmidt has received personal fees from Boston Scientific and from Abbot Vascular, outside the submitted work; K. Stein is an employee and shareholder at BSC; L.V. Boersma has received personal fees from Boston Scientific and from Medtronic, outside the submitted work.
- –
Elderly patients with atrial fibrillation have greater risks of both cardioembolic events and major bleeding than younger patients.
- –
LAAO offers nonpharmacological stroke protection, obviating the need for oral anticoagulation.
- –
LAAO could be an attractive alternative to oral anticoagulation in elderly patients.
- –
This study shows the safety and efficacy of LAAO in very old patients.
The following investigators and institutions participated in the EWOLUTION registry. Investigators are listed after centers in alphabetical order.
Al Qassimi Hospital: Arif Al Nooryani; Asklepios Klinik Saint Georg: Felix Meincke; Asklepios Klinik Weissenfels: Thomas Fiedler; Ospedale di Cirie: Gaetano Senatore; Beaumont Hospital: David Foley; Cardioangiologisches Centrum Bethanien: Boris Schmidt; CHRU de Lille: François Brigadeau; CHU Grenoble Hopital Michallon: Pascal Defaye; CHU Henri Mondor: Emmanuel Teiger; CHU La Timone Hospital: Jean-Louis Bonnet; Dominikus- Krankenhaus: Christof Wald; Elisabeth Krankenhaus Essen: Thomas Schmitz; Erasmus MC - University Medical Center Rotterdam: Tamas Szili-Torok; Evangelisches Krankenhaus Bielefeld: Wladimir Tschishow; Fondazione Centro San Raffaele: Patrizio Mazzone; Freeman Hospital: David Crossland; Herzkatheter Asklepios Wandsbek: Martin W. Bergmann; Hôpital Bichat: Alec Vahanian; Hospital Clínico Salamanca: Ignacio Cruz-Gonzalez; Hôpital du Haut Lévêque: Jean-Benoit Thambo; Johannes Gutenberg Universität Mainz: Tommaso Gori; John Radcliffe Infirmary Oxford II: Timothy Betts; King Fahed Medical City Prince Salman Cardiac Center: Faisal Al Smadi; Klinikum Neuperlach: Harald Mudra; Krankenhaus Barmherzige Bruder: Robin Molitoris; Medisch Centrum Leeuwarden: Richard Folkeringa; Medisch Spectrum Twente: Yorick Stevenhagen; NCN Nouvelles Cliniques Nantaises: Daniel Gras; Onze Lieve Vrouw Ziekenhuis: Tom De Potter; Ospedale Ferrarotto: Corrado Tamburino; Ospedale Sacro Cuore Don Calabria: Giulio Molon; Regional Vascular Center: Vladimir Protopopov; Royal Victoria Hospital: Mark Spence; University Hospital of Lord's Transfiguration Poznan: Marek Grygier; Santa Maria: Eduardo Infante Oliveira; St Antonius Ziekenhuis: Lucas Boersma; St Katharinen Krankenhaus: Horst Sievert; State Cardiology Research Center: Evgeny Merkulov; State Research Institute of Circulation Pathology: Evgeny Pokushalov; Szpital Uniwersytecki: Adam Sukiennik; The Brompton Hospital: Tom Wong; Universitätsmedizin Greifswald: Mathias Busch; Universitätsmedizin Berlin - Charité Virchow Standort: Leif-Hendrik Boldt; UniversitätsKlinikum Bonn: Georg Nickenig; Universität Leipzig: Martin Neef; Vivantes Klinikum Am Urban: Hüseyin Ince; Vivantes Klinikum im Friedrichshain: Stephan Kische.