Left ventricular noncompaction is a poorly defined and controversial entity, with wide phenotypic expression: from a simple anatomical trait to a disease with overt cardiac affection. Current diagnostic criteria rely exclusively on morphologic features of hypertrabeculation, which have low specificity for identifying true cardiomyopathy cases. The management of left ventricular noncompaction is also heterogeneous, and there are no dedicated clinical practice guidelines. The most common cardiovascular complications are heart failure, ventricular arrhythmias, and systemic embolisms. In this review, we discuss the diagnostic limitations of the available criteria, and propose a comprehensive alternative approach (including functional imaging variables, tissue characterization, genetics, and family screening) that may help in the differential diagnosis of hypertrabeculation cases. We also describe the genetic background of the disease and discuss the overlap with other cardiomyopathies. Finally, we focus on controversial issues in clinical management and suggest the use of the previously-mentioned variables for risk stratification and for individualization of patient follow-up.
Keywords
Left ventricular noncompaction (LVNC) is a heterogeneous entity characterized by prominent left ventricular (LV) trabeculae, deep intertrabecular recesses, and a thin compacted myocardial layer.1 Even though it was first described more than 30 years ago,2 LVNC is still a poorly defined and understood disorder, considered a nonclassified familial cardiomyopathy by the European Society of Cardiology,3 and a genetic cardiomyopathy by the American Heart Association.4
Several diagnostic imaging parameters have been proposed, but there are no standardized criteria. The different criteria are inconsistent, and focus only on morphologic traits, which has resulted in an overdiagnosis of LVNC.5 Additionally, this heterogeneity has led to highly variable LVNC outcomes being reported in the literature. All in all, no clinical practice guidelines are available for such a controversial entity, which makes clinical management highly challenging.
In this review, we focus on the various available diagnostic criteria and the complexity of reaching a correct diagnosis. In addition, we discuss the difficulties and controversies of specific clinical management.
DIAGNOSTIC CRITERIA AND DIFFERENTIAL DIAGNOSISDefinition of the entityLVNC diagnosis is currently based on cardiac imaging techniques, both with transthoracic echocardiography (TTE) and cardiovascular magnetic resonance (CMR). Different diagnostic criteria have been proposed, which focus on morphologic traits to compare the compacted and noncompacted myocardial layers, without considering functional parameters. These criteria are inconsistent, not standardized, and based on small series of patients, which makes the diagnosis of LVNC widely heterogeneous (table 1).
Summary of different diagnostic criteria for left ventricular noncompaction
| Criteria | Chin | Jenni | Stöllberger | Petersen | Jacquier | Stacey | Grotthoff | Captur |
|---|---|---|---|---|---|---|---|---|
| Reference | 2 | 6 | 7, 8 | 9 | 10 | 11 | 12 | 13 |
| Number of patients | 8 | 7 | 62 | 7 | 16 | 122 | 12 | 30 |
| Gold-standarddiagnosis | Necropsy, 3 (38) | Anatomical examination, 7 100) | > 3 trabeculations protruding from the LV wall and intertrabecular spaces | TTE or CMR evidence of a 2 -layered myocardium and 1 additional feature | Jenni criteria | Jenni and Petersen criteria | Jenni criteria and 1 additional feature | Jenni criteria and 1 additional feature |
| Age, y | 9 [11-22] | 39±17 | 50 [18-75] | 29±13 | 48±17 | 57±17 | 35±18 | 41±13 |
| Females | 3 (38) | 2 (29) | 13 (21) | 2 (29) | 6 (38) | 50 (41) | 9 (75) | 14 (47) |
| Left ventricular systolic function | FS range 10%-33% | LVEF 29%±6% | FS <30% in 43 (69) patients | LVEF 53%±17% | Not reported | LVEF 44%±16% | LVEF 51%±16% | LVEF 52%±17% |
| Cardiovascular events | 5 (63) | 7 (100) death and/or heart transplant | 45 (73) heart failure | 3 (43) | Not reported | 36 (30) heart failure | 6 (50) ventricular tachycardia | 20 (67) |
| Imaging technique | TTE | TTE | TTE | CMR (1.5 T) | CMR (1.5 T) | CMR (1.5 T) | CMR (1.5 T) | CMR (1.5 T) |
| View | Short-axis | Short-axis | Apical views | Longitudinal-axis | Short-axis stack | Short-axis | Short-axis stack | Short-axis stack |
| Cardiac cycle phase | End-diastole | End-systole | End-diastole | End-diastole | End-diastole | End-systole | End-diastole | End-diastole |
| Trabeculation measurement | Distance between the epicardial surface and trough of a trabecular recess (X) and the distance between the epicardial surface and peak of the trabeculation (Y) | Measurement of the wall thickness of the NC and C myocardial layers | Presence of more than 3 trabeculations protruding from the left ventricular wall, apically to the papillary muscles, visible in 1 image plane and measurement of the wall thickness of the NC and C myocardial layers | Measurement of the wall thickness of the NC and C myocardial layers | Semiautomatic outlining of endocardial and epicardial contours, including papillary muscles | Measurement of the wall thickness of the NC and C myocardial layers | Manual tracing of epicardial borders in 4-chamber and vertical long-axis views, propagation algorithm in short-axis and manual tracing of trabeculations | Segmentation of the endocardial border and box-counting method for fractal analysis |
| Cutoff point | X/Y ≤ 0.5 | NC/C ≥ 2 | NC/C ≥ 2 | NC/C ≥ 2.3 | Trabeculated mass> 20% of the total LV mass | NC/C ≥ 2 | Trabeculated mass> 15 g/m2 or> 25% of the total LV mass or NC/C ≥ 3 | Maximal apical fractal dimension ≥ 1.30 |
| Interobserver variability | Significant at apical segments, insignificant at mid and basal segments | Not reported | Not reported | Not reported | Intraclass correlation coefficient 0.95 (95%CI, 0.89-0.97) | Spearman correlation 0.82 and 0.78 for the compacted and noncompacted layers, respectively | Not reported | Intraclass correlation coefficient 0.96 (95%CI 0.93-0.97) |
| Figure | 1A | 1A | 1B | 2A | 2B | 2C |
95%CI, 95% confidence interval; C, compacted; CMR, cardiovascular magnetic resonance; FS, fractional shortening; LVEF, left ventricular ejection fraction; NC, noncompacted; TTE, transthoracic echocardiography.
Unless otherwise indicated, the data are expressed as No. (%), mean±standard deviation, or median [interquartile range].
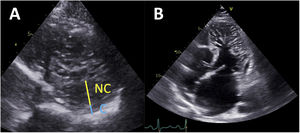
Chin et al.2 first reported LVNC TTE diagnostic criteria based on a series of 8 patients. These authors proposed measuring the distance from the epicardial surface to the trough of the trabeculae (X), and the distance from the epicardial surface to the peak of the trabeculae (Y) on short-axis views. The authors defined that a ratio of X/Y ≤ 0.5 at end-diastole was suggestive of LVNC (figure 1A). Later, Jenni et al.2 published a series of 34 patients, and suggested measuring the compacted (C) epicardial layer and the noncompacted (NC) endocardial layer. A ratio of NC/C ≥ 2 measured on short-axis views at end-systole was considered to be consistent with LVNC6 (figure 1A). The third relevant TTE criterion was reported by Stöllberger et al.2 in a series of 62 patients. Based on their observations, the presence in 1 apical plane of more than 3 trabeculations protruding from the LV wall (apically from the insertion of the papillary muscles) was considered suggestive of LVNC7 (figure 1B). This criterion was later updated to also include a ratio of NC/C ≥ 2 measured on short-axis views at end-diastole.8 Of all these proposed criteria, Jenni's criterion has become the most widely used in clinical practice with TTE.
Left ventricular noncompaction diagnostic criteria with transthoracic echocardiography. A: measurement of the wall thickness of the compacted (C) and noncompacted (NC) myocardial layers on a short-axis view, at end-diastole (Chin criteria) or end-systole (Jenni Criteria). B: apical 4-chamber view showing numerous and prominent trabeculations protruding from the left ventricle apically from the papillary muscles (Stöllberger criteria).
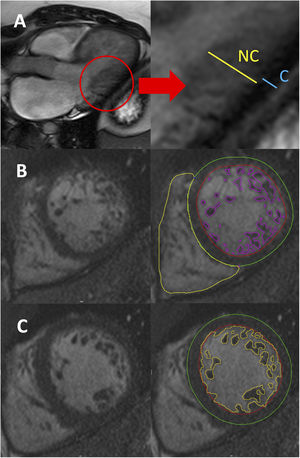
With CMR, at least 5 different diagnostic criteria have been described, although only 2 of them (Petersen and Jacquier) are usually applied in clinical practice. Petersen et al.9 first reported CMR-derived diagnostic criteria in a series of 7 patients who showed hypertrabeculation on TTE and/or CMR. The authors reported that a ratio of NC/C layers> 2.3 measured on long-axis views at end-diastole was suggestive of LVNC (figure 2A). Jacquier et al. later published other criteria based on a series of 16 patients meeting Jenni's TTE criteria. According to their observations, a trabeculated mass> 20% of the total LV mass showed an excellent diagnostic accuracy for LVNC10 (figure 2B). The criteria of Jacquier et al. seem more robust than Petersen's criteria considering that the global hypertrabeculated myocardium is measured and not only the ratio of hypertrabeculation in a specific region. Even though Jacquier's criteria have excellent interobserver reproducibility, their assessment is time-consuming. Therefore, Petersen's criteria have become the most widely used in routine clinical practice. Indeed, due to increased spatial resolution and better tissue characterization compared with TTE, CMR is always recommended to confirm LVNC diagnosis.
Left ventricular noncompaction diagnostic criteria with cardiovascular magnetic resonance. A: measurement of the wall thickness of the compacted (C) and noncompacted (NC) myocardial layers on a longitudinal-axis view at end-diastole (Petersen criteria). B: endocardial (red) and epicardial (green) contours, including semiautomatic outlining of the trabeculae (purple) for trabeculated mass quantification, in a short-axis view (Jacquier criteria). C: endocardial (red) and epicardial (green) contours, including the automatic outlining of the trabeculae (yellow) for fractal analysis, in a short-axis view (Captur criteria).
Some other CMR diagnostic approaches have been described. Stacey et al.11 proposed measuring the NC/C ratio in short-axis cine sequences at end-systole. Based on a series of 122 patients, they described that a ratio ≥ 2 was suggestive of LVNC. Grothoff et al.12 published a small series of 12 LVNC patients and concluded that a cutoff point of 15 grams of trabeculated mass/m2 showed an excellent diagnostic accuracy for LVNC. Finally, Captur et al.13 published a series of 30 patients and described a new diagnostic method based on fractal analysis, a semiautomatic tool to quantify the degree of hypertrabeculation. Fractal dimension, a marker of geometric complexity, is obtained after contouring the LV endocardium at end-diastole, and a maximal apical fractal dimension ≥ 1.30 is diagnostic of LVNC (figure 2C). Fractal analysis has excellent inter- and intraobserver reproducibility, although its use has not been widely adopted because of the limited availability of the software needed for its assessment.
Limitations of the current definitionAs previously mentioned, none of these criteria include functional LV parameters such as LV size, systolic function (LV ejection fraction, left ventricular ejection fraction [LVEF]), or the presence of late gadolinium enhancement (LGE) (figure 3). This has probably resulted in an overdiagnosis of LVNC cases,5 even in asymptomatic patients with no established cardiovascular disease: in a population-based study, 15% fulfilled at least 1 LVNC criteria.14 It should also be noted that the sole presence of hypertrabeculation fulfilling LVNC criteria has not been associated with either long-term LV remodelling15 or outcomes.16,17 A substudy of the MESA registry analyzed 2742 patients with a baseline CMR and a follow-up at 9.5 years. Patients were divided into quintiles of hypertrabeculation following Petersen's assessment,9 and no differences were found among groups in terms of changes in LV volumes or LVEF.15 When considering clinical endpoints, a study of 700 patients referred to CMR found that fulfilling any of the 4 aforementioned CMR criteria was not associated with major adverse cardiovascular events (MACE) at 7 years of follow-up.16 Similar findings were observed in a large meta-analysis of 574 patients with LVNC: diagnostic criteria per se had no prognostic value beyond LVEF or the presence of LGE.17 In addition, hypertrabeculation in nonischemic dilated cardiomyopathy (DCM) has not been associated with a worse outcome.18
Furthermore, there have been reports of acquired and reversible hypertrabeculation. In particular, a high prevalence has been observed in athletes: 8% to 10% of highly-trained athletes fulfil LVNC criteria.19,20 Indeed, a large population-based study showed that vigorous physical activity was progressively associated with hypertrabeculation: individuals undertaking more activity showed a higher proportion of LVNC criteria, irrespective of LV volumes.21 In addition, another study showed that pregnancy was frequently associated with hypertrabeculation: 8% of women developed LVNC during pregnancy, which mostly disappeared after childbirth.22 Similarly, a large proportion of patients with sickle cell anemia were found to have hypertrabeculation, and at least 8% fulfilled LVNC criteria.23 These findings suggest that certain changes in loading conditions might lead to physiological remodeling with marked LV hypertrabeculation without prognostic implications.
In contrast, some other studies have shown discordant results and have suggested that hypertrabeculation might not be considered a benign finding, reinforcing the fact that LVNC is a highly heterogeneous entity. In a large population-based study of 10 097 patients who underwent cardiac computed tomography, patients with hypertrabeculation were at increased risk of MACE at 4.0 years of follow-up, and the degree of hypertrabeculation (measured as the indexed trabeculated LV mass) was independently associated with outcomes.24 Similarly, another study of 339 LVNC patients followed up for 6.3 years showed that LV hypertrabeculation (defined by Petersen's criteria) extending from the apex to mid-basal segments was independently associated with all-cause mortality.25 In addition, in a series of 328 patients with LVNC followed up for 3.1 years, the authors found that the presence of myocardial thinning (defined as an abrupt thinning of compacted myocardium by 50% or greater compared with a contiguous segment) was independently associated with a higher risk of MACE.26
Little is known about the involvement of the right ventricle (RV) in LVNC. The RV is a naturally more hypertrabeculated chamber than the LV and therefore applying the same LV criteria might lead to a considerable overlap with the healthy population. Specific cutoff values have not been published or validated, and quantification of RV trabeculae with TTE is technically challenging. Several CMR measurements of the RV hypertrabeculation have been proposed, including the RV end-diastolic trabeculated area and volumes, as well as the RV NC/C ratios in short-axis and 4-chamber views.27 However, a strong overlap with controls has been observed and, without prognostic implications, caution is recommended when analyzing these variables.
One step further: the holistic approachAll this controversial data has prompted some authors to question whether LVNC is a true cardiomyopathy or a simple morphologic trait.28,29 Consequently, some algorithms combining LVEF, LGE presence, electrocardiogram data, family history, or genetic testing, have been proposed to differentiate physiologic hypertrabeculation (a phenotypic trait) from pathologic forms30–32 (table 2). In a recent large multicentre study, a step-wise algorithm has been proposed to identify low-risk LVNC patients: cases with no electrocardiogram abnormalities, with a preserved LVEF, no LGE, and no family history did not show MACE at 5 years of follow-up. This suggests that those cases might correspond to physiologic or “benign” hypertrabeculation rather than a cardiomyopathy. However, patients with any of the aforementioned abnormalities presented an increased risk of cardiovascular events, confirming the pathological nature of such hypertrabeculation.33
Differential diagnosis of patients with morphologic features of hypertrabeculation
| Favors cardiomyopathy | Variable | Favorsphenotypic trait |
|---|---|---|
| Dilated and/orhypertrophic LV | LV dimensions | Nondilated andnonhypertrophic LV |
| Reduced | LVEF | Preserved |
| Abnormal (even if pEF) | GLS | Normal |
| Abnormal (even if pEF) | Diastolic function | Normal |
| + | LGE | - |
| Elevated | ECV | Normal |
| + | Past family history and/or family aggregation | - |
| + | Genotype | - |
| Shortness of breath,syncope, palpitations... | Symptoms | Asymptomatic |
| Heart failure, supraventricular/ventricular arrhythmias, systemic embolisms, death | Cardiovascular events | No events |
| LBBB, T wave inversion... | ECG | Narrow QRS, no repolarization abnormalities |
| Elevated | Biomarkers(NT-proBNP, hs-cTn) | Normal |
| Non-sustained VT, frequent ventricular ectopic beats.. | Holter | Normal |
| Inducible VT, decrease in LVEF at peak stress | Treadmill test / stress TTE | Normal |
| Progressive LV dilatation/dysfunction | Change inLV dimensions / LVEF | No significant changes in LV dimensions or LVEF |
ECG, electrocardiogram; ECV, extracellular volume; GLS, global longitudinal strain; hs-cTn, high-sensitivity cardiac troponin; LBBB, left bundle branch block; LGE, late gadolinium enhancement; LV, left ventricle; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; pEF, preserved ejection fraction; TTE, transthoracic echocardiography; VT, ventricular tachycardia.
Additionally, other imaging parameters, such as deformation and mapping variables have been proposed, but they have only been tested in small case series and are not recommended in routine clinical practice. Global strain, LV rotation, and torsion on TTE are impaired in LVNC even with a preserved LVEF, and significantly lower compared with controls.34 Similar results have been described with CMR with a good correlation with the degree of hypertrabeculation, and with incremental diagnostic value.35,36 In addition, native T1 and extracellular volume are higher in LVNC patients compared with controls, even in the absence of LGE.37
Genetic tests are also very useful in the diagnosis of pathologic forms of LVNC. Even though 189 genes have been reported in relation to LVNC, only 32 have been found to be significantly associated with the entity in a recent review (list on table 3).38 Genetic variants usually involve sarcomeric genes (between one third and half of the cases, most often MYH7, MYBPC3, ACTC1 and TTN), but may also affect transcriptional/translational regulators, mitochondrial and cytoskeleton genes,38,39 ion channelsn40 and copy number variations.41 Most of the genetic causes are missense mutations (55%), and the most frequent inheritance pattern is autosomal dominant (83%), while X-linked and mitochondrial patterns are less prevalent.39 Thus, if there is clinical suspicion of LVNC, genetic testing is reasonable to confirm the diagnosis, as recommended by an expert consensus document with a class IIB recommendation.42 The presence of a pathogenic or likely pathogenic genetic variant will confirm the diagnosis, avoiding unnecessary examinations in genotype-negative relatives. However, pathogenic variants are described in approximately 30% to 40% of cases only.33,43,44 Most importantly, certain genotypes have been associated with phenotype and prognosis: specifically, MYBPC3 and TTN variants, as well as other genes, correlate with a higher risk of MACE, while MYH7 and ACTC1 variants have more favorable outcomes.39
List of genes associated with left ventricular noncompaction. Only genes with a definitive or moderate association with left ventricular noncompaction according to a recent review 38 are shown in the table
| Gene function | Gene | Gene name |
|---|---|---|
| Sarcomere | ACTC1 | Actin alpha cardiac muscle 1 |
| DES | Desmin | |
| LDB3 | LIM domain binding 3 | |
| MYBPC3 | Myosin binding protein C3 | |
| MYH7 | Myosin heavy chain 7 | |
| PLN | Phospholamban | |
| OBSCN | Obscurin | |
| RYR2 | Ryanodine receptor 2 | |
| TNNT2 | Troponin T2, cardiac type | |
| TPM1 | Tropomyosin 1 | |
| TTN | Titin | |
| Cytoskeleton | DMD | Dystrophin |
| DTNA | Dystrobrevin alpha | |
| LMNA | Lamin A/C | |
| Desmosome | PKP2 | Plakophilin 2 |
| Intracellular trafficking | LAMP2 | Lysosomal associated membrane protein 2 |
| PLE-KHM2 | Pleckstrin homology and RUN domain containing M2 | |
| Ion channel | HCN4 | Hyperpolarization activated cyclic nucleotide gated potassium channel 4 |
| SCN5A | Sodium voltage-gated channel alpha subunit 5 | |
| Mitochondria | NNT | Nicotamide nucleotide transhydrogenase |
| TAZ | Tafazzin | |
| TMEM70 | Transmembrane protein 70 | |
| Protein degradation | MIB1 | Mindbomb E3 ubiquitin protein ligase 1 |
| Signal transduction | ALPK3 | Alpha kinase 3 |
| DMPK | DM1 protein kinase | |
| Transcriptional/translational regulator | NKX2.5 | NK2 homebox 5 |
| NONO | Non-POU domain containing octamer binding | |
| PRDM16 | PR/SET domain 16 | |
| RBM20 | RNA binding motif protein 20 | |
| TBX20 | T-box transcription factor 20 | |
| TBX5 | T-box transcription factor 5 |
DM1, myotonic dystrophy 1; LIM, acronym of LIN-11, Isl-1 and MEC-3; RNA, ribonucleic acid.
However, LVNC shares a common genetic background with other cardiomyopathies such as DCM and hypertrophic cardiomyopathy (HCM), which usually also involve sarcomeric genes.28,38,39 This finding explains the large overlap among all these cardiomyopathies: some patients with DCM or HCM also meet the diagnostic criteria for LVNC, while patients with definite LVNC may also display features of DCM or HCM,1 even with different phenotypes among relatives.45 Some authors have proposed that there is a continuum of phenotypical expression between LVNC, DCM, and HCM, and that both genetic factors and nongenetic triggers interact with the final phenotype.38,46 However, no clear features have been proposed to differentiate these phenotypes in clinical practice, which often makes imaging-based diagnosis challenging. LVNC may also occur in association with congenital heart diseases or with neuromuscular disorders.28 In addition, cases of isolated LVNC with no phenotypic features of other cardiomyopathies have been described in infantile taffazinopathies (eg, Barth syndrome)47 or in mutations of the NOTCH pathway regulator MIB1 gene.48 Indeed, genetic pathways that are uniquely associated with LVNC and not DCM or HCM are often involved in cardiomyocyte development and differentiation, such as the NOTCH pathway.38 This finding supports the hypothesis that LVNC may be a consequence of an abnormal embryogenesis.
Some cardiac imaging studies have attempted to define markers that allow differentiating between different cardiomyopathies. A characteristic deformation pattern on TTE, with higher values in the LV base compared with the apex (where hypertrabeculation is more pronounced) could differentiate LVNC from DCM, which shows uniformly reduced strain values.49 The combination of fractal analysis and global longitudinal strain on CMR allowed accurate differentiation of LVNC from DCM patients.50 Recently, a study based on radiomics (an emerging imaging analysis technique for deeper phenotyping in CMR) showed that artificial intelligence allowed excellent differentiation of DCM, HCM, and LVNC in an automatic and highly-efficient way.51
Finally, family aggregation has been described in approximately 40% to 50% of LVNC cases,33,43,45 and therefore family screening is recommended in all patients to confirm the index diagnosis and to identify asymptomatic relatives.52 According to expert consensus,42 if a disease-causing genetic variant is described in a patient, first-degree relatives should undergo clinical and genetic assessment with a class I recommendation.
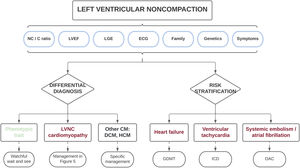
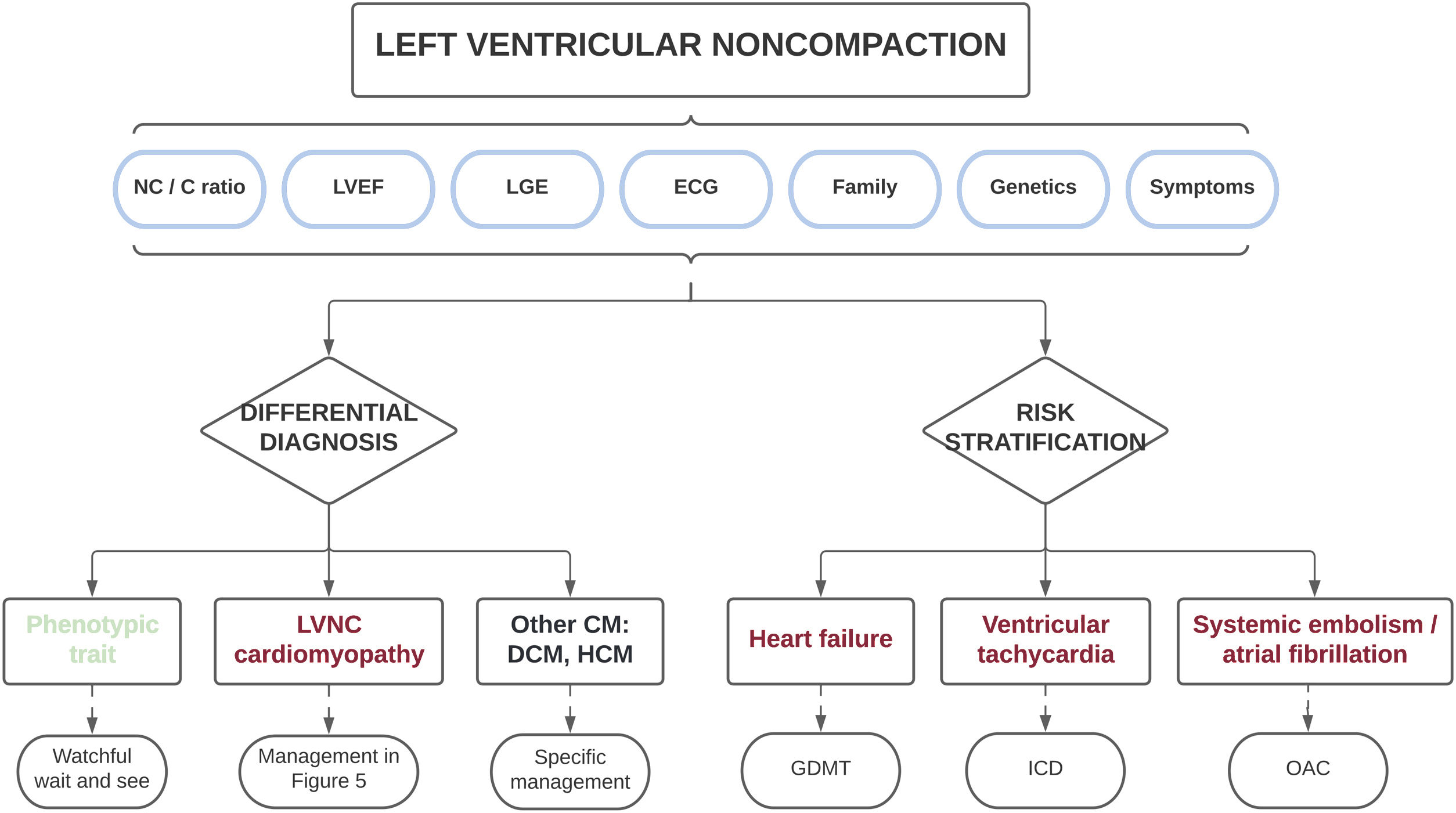
Therefore, based on the different published series collected in this review, the presence of a marked myocardial hypertrabeculation should not be considered, a priori, as a normal morphological trait and should instead be studied in an integral manner. Most importantly, the diagnosis should not only be based on morphologic imaging criteria, but should also consider clinical status, the electrocardiogram, family history, genetic testing, functional imaging variables, and the presence of myocardial fibrosis, among other parameters. Such a holistic approach will allow for the differentiation of physiologic hypertrabeculation cases from those with true LVNC cardiomyopathy (table 2). Only those patients with morphological criteria who do not show other red flags can be considered as a normal variant, whereas only those with definite pathologic features (electrocardiogram changes, reduced LVEF, LGE and/or family aggregation) should be diagnosed with LVNC. This differential diagnosis is clinically relevant because individuals with a simple phenotypic trait have favorable outcomes and might not require periodic follow-up, avoiding unnecessary costs and the psychological burden of an incorrect diagnosis. In contrast, patients with cardiomyopathy are at increased risk of developing cardiovascular events, and should therefore be carefully monitored and managed appropriately (see next section) (figure 4).
Central illustration. Clinical approach in individuals with morphologic features of left ventricular noncompaction. A comprehensive diagnostic evaluation is recommended to exclude patients with a simple phenotypic trait and those with other cardiomyopathies. The same variables used in the differential diagnosis may be applied for risk stratification to individualize patient treatment and follow-up. C, compacted; CM, cardiomyopathy; DCM, dilated cardiomyopathy; ECG, electrocardiogram; GDMT, guideline-directed medical therapy; HCM, hypertrophic cardiomyopathy; ICD, implantable cardioverter-defibrillator; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; LVNC, left ventricular noncompaction; NC, noncompacted; OAC, oral anticoagulation.
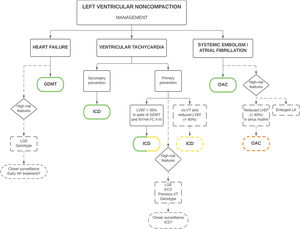
Because of the lack of universal diagnostic criteria, outcomes in LVNC are uncertain. Some series confer LVNC a poor prognosis,2,44,53 while others suggest a more benign profile.15,16,54 Additionally, there are no specific clinical practice guidelines, which contributes to the heterogeneous management of these patients. However, some expert consensus recommendations are available,42 with subtle changes in clinical management compared with other cardiomyopathies (figure 5), reinforcing the importance of a correct diagnosis.
Clinical management of patients with left ventricular noncompaction. Green stands for a class I recommendation, yellow for a class IIa recommendation and orange for a class IIb recommendation. ECV, extracellular volume; ICD, implantable cardioverter-defibrillator; GDMT, guideline-directed medical therapy; HF, heart failure; ns, non-sustained; LA, left atrium; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; NYHA FC, New York Heart Association Functional Class; VT, ventricular tachycardia; OAC, oral anticoagulation.
The main complications associated with LVNC are heart failure (HF), ventricular arrhythmias, systemic embolisms, and death. Some patients may also be asymptomatic, diagnosed incidentally or during family screening. The most common clinical manifestation of this disease is HF, which is found in 14% to 21% of adult patients.26,33,43 The incidence is higher than that observed in the DCM population (4.05 events per 100 person-years).33,55 LVEF is the strongest predictor of HF, and patients with reduced LVEF are at increased risk, especially those with an LVEF ≤ 35%.33 Thus, patients with reduced LVEF should be promptly treated with guideline-directed medical therapy to reduce the risk of death and HF hospitalization and to improve clinical and functional status with a class I recommendation according to international guidelines56,57 (figure 5). In addition, the presence of LGE (a marker of myocardial fibrosis on CMR), has been associated with HF risk, even in the absence of severe systolic dysfunction.33 Other variables that correlate with HF are a higher degree of hypertrabeculation24 and certain genotypes, specifically TTN33,39 and MYBPC339 variants. Therefore, it seems reasonable that these patients undergo closer clinical surveillance (including natriuretic peptides and echocardiography) to detect early symptoms and signs of HF. However, prospective studies should clarify whether early initiation of HF treatment would improve prognosis (figure 5). At this stage, the recommended management is comparable to that of patients with HF and reduced LVEF.56,57
Ventricular arrhythmias are another frequent and feared complication, being present in 19% to 21% of patients,26,33 with sudden cardiac death (SCD) in 5% to 6%.58,59 A multicenter retrospective study estimated the incidence of arrhythmic events at around 2.79 events per 100 person-years,33 which is statistically similar to the numbers reported for DCM.55 LVEF remains the strongest predictor of ventricular arrhythmias,33 and indications for primary prevention of SCD and implantable cardioverter-defibrillator (ICD) implantation are comparable to those in the general population: patients with an LVEF ≤ 35% despite optimal medical therapy for ≥ 3 months, in New York Heart Association functional class II and III, and expected survival longer than 1 year, with a class IIa recommendation in the latest European guidelines, and with a class I recommendation in the American guidelines.56,57,60,61 The weaker European recommendation in nonischemic patients was changed after the results of the DANISH trial.62 Additionally, an expert consensus document recommends prophylactic ICD in cases of reduced LVEF and nonsustained ventricular tachycardias, even in the absence of LGE, with a class IIa recommendation42 (figure 5). Indications for secondary prevention are more homogeneous in different guidelines: patients surviving an SCD, and those with documented ventricular fibrillation or hemodynamically not tolerated/sustained ventricular tachycardias should receive an ICD with a class I recommendation if expected survival is longer than 1 year, according to international guidelines (figure 5).56,57,60,61 It is worth mentioning that high rates of appropriate ICD shocks have been reported in LVNC patients, after both primary and secondary prevention,44,63,64 and consequently it seems to be an effective therapy in this cardiomyopathy. Therefore, a low threshold for ICD implantation is suggested, even if evidence of its benefits in nonischemic etiology is still unclear.
LGE is another important predictor of ventricular arrhythmias in LVNC,17,33,65 and myocardial fibrosis seems to be an arrhythmic substrate. Consistent with other cardiomyopathies,55,66,67 LGE has been associated with poor outcomes and SCD risk in LVNC, even in the absence of severe systolic dysfunction.33 However, LGE has not yet been incorporated into clinical practice guidelines, which are solely based on LVEF.56,57,60,61 In a small study, extracellular volume on CMR was also associated with the risk of ventricular arrhythmias even in the absence of LGE.37 In addition, the presence of preceding arrhythmias, including ventricular tachycardias, has been correlated with an increased risk of SCD in a pediatric LVNC population.59 Finally, certain genotypes have also been associated with ventricular arrhythmias: ACTC1,33MYBPC3, arrhythmogenic genes (ABCC9, ANK2, CACNA2D1, CASQ2, HCN4, KCNE3, KCNH2, KCNQ1, RYR2, and SCN5A), and nonarrhythmogenic nonsarcomere genes (DMPK, DSP, DTNA, FKTN, HFE, JUP, LMNA, PKP2, PLEC, PLN, PRDM16, RBM20, and SGCD).39
All of these risk factors identify patients at increased risk of arrhythmic events. Therefore, closer clinical surveillance and proactive detection of subclinical arrhythmias, including Holter monitoring and treadmill tests, is recommended. At present, no recommendations can be made on early ICD implantation in these patient subgroups, and future prospective studies should investigate whether a more aggressive approach might improve clinical outcomes (figure 5). A combined approach with LVEF and LGE, similar to that proposed in DCM patients, might be considered to identify high-risk patients.68
Systemic embolisms are another classic manifestation commonly associated with LVNC, with an estimated prevalence of approximately 15%.69 However, recent series have reported a lower risk around 3.1% to 4.6%,26,33 which could be explained by more aggressive anticoagulation therapy. Beyond traditional embolic factors such as atrial fibrillation (AF) and systolic dysfunction, LVNC has an intrinsic embolic risk. This has been considered to be secondary to blood stasis in the intertrabecular recesses, even though a greater degree of hypertrabeculation has not been associated with a higher risk of stroke.24 The presence of previous systemic embolisms in LVNC is an indication for oral anticoagulation therapy (OAC) with a class I recommendation according to an expert consensus (figure 5).42
AF is also common in LVNC, with a prevalence of up to 29% of patients,70 and is associated with an increased risk of systemic embolism.69 In patients with LVNC, AF is an indication for OAC irrespective of thromboembolic risk assessed by traditional scores (CHA2DS2-VASc), with a class I recommendation according to the European guidelines and expert consensus (figure 5).42,71 Left atrial dilatation has also been associated with systemic embolisms risk in LVNC,33 which could be an early marker of AF. Although no recommendations for the use of prophylactic OAC can be made based on left atrial dimensions, patients with enlarged left atria might be considered to undergo a more proactive search for subclinical AF, as has been suggested in HCM.72 A reduced LVEF is another consistent risk factor for SE,33,69 and patients with an LVEF ≤ 40% might be considered for prophylactic OAC, even in sinus rhythm, with a class IIB recommendation according to an expert consensus document (figure 5).42 Based on the increased embolic risk, proactive screening of intraventricular thrombi with contrast echocardiography is recommended if there is reduced LVEF. If a thrombus is detected, vitamin K antagonists are usually the first choice treatment due to the lower embolic risk compared with direct oral anticoagulants.73 Otherwise, direct oral anticoagulants are usually preferred over vitamin K antagonists, owing to a better safety profile, even though their evidence in LVNC is limited to case reports.74 Recently, an algorithm has been proposed for prophylactic OAC in LVNC, which includes all the aforementioned variables.75
Table 4 describes the most important LVNC series discussed in this review.
Summary of the main left ventricular noncompaction studies cited in the review
| First author | Number of patients | Women, n (%) | Age, y | Diagnostic criteria | LVEF, % (SD or IQR) | Positive genotype | Family aggregation | Follow-up, y | Heart failure | Ventricular arrhythmias | Systemic embolisms | Death | MACE, n (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stacey, 201311 | 122 | 50 (41) | 57±17 | Stacey | 44±16 | Not reported | Not reported | Not reported | 36 (30) | 6 (5) | 10 (8) | 6 (5) | Not reported |
| Vaidya, 202125 | 339 | 157 (46) | 47 [34-61] | Jenni, Chin and Petersen | 45 [30-58] | Not reported | Not reported | 6.3 [3.1-10.8] | Not reported | Not reported | Not reported | 59 (17) | Not reported |
| Ramchand, 202126 | 328 | 136 (42) | 43±17 | Petersen | 45±14 | Not reported | Not reported | 3.1 | 41 (13) | 70 (21) | 15 (5) | 15 (5) | 102 (31) |
| Casas, 202133 | 585 | 251 (43) | 45±20 | Jenni and Petersen | 48±17 | 99 (42) | 106 (42) | 5.1 [2.3-8.1] | 110 (19) | 87 (15) | 18 (3) | 34 (6) | 223 (38) |
| van Waning, 201843 | 327 (275 adults) | 152 (46) | 45 [33-56] | Jenni and Petersen | 174 (53) with LV systolic dysfunction | 104 (32) | 120 (37) | 2.1 [0.3-4.8] in adults | 72 (22) | 19 (6) | 38 (12) | 24 (7) | 72 (22) |
| Sedaghat-Hamedani, 201744 | 95 | 20 (19% of index patients) | 41±14 | Stöllberger, Jenni and Petersen | 38±15 | 36 (38) | 22 (23) | 5.1 | Not reported | 24 (35) | 7 (10) | 9 (13) | Not reported |
| Brescia, 201359 | 242 children | 97 (40) | 9 [0-14] | Jenni | 150 (62) with LV systolic dysfunction | Not reported | 56 (23) | 4.0 [1.8-15.9] | Not reported | 42 (17) | Not reported | 31 (13) | Not reported |
| Andreini, 201665 | 113 | 43 (38) | 44±17 | Jenni and Petersen | 45±15 | Not reported | 17 (15) | 3.8 [2.2-5.7] | 16 (14) | 10 (9) | 5 (4) | 5 (4) | 36 (32) |
| Stöllberger, 201169 | 144 | 43 (30) | 54±16 | Stöllberger | 88 (61) with LV FS <25% | Not reported | Not reported | Not reported | Not reported | Not reported | 22 (15) | Not reported | Not reported |
FS, fractional shortening; IQR, interquartile range; LV, left ventricular; LVEF, left ventricular ejection fraction; MACE, major adverse cardiovascular events; SD, standard deviation.
The data are presented as No. (%), mean±standard deviation, or median [interquartile range].
All in all, it seems clear that the incidence of MACE in patients with confirmed LVNC (determined not only by morphologic traits) is not negligible. In an attempt to establish risk scores in this population, our group conducted a large multicenter retrospective study of 585 patients, of whom 223 (38%) experienced MACE during a median follow-up of 5.1 years. On multivariate analysis, the variables independently associated with MACE were age, LVEF, and the presence of electrocardiogram changes. These variables, combined with sex, family aggregation, and cardiovascular risk factors were incorporated to develop a risk prediction model that was also validated in an external population. The performance of the score was very high (C-index 0.72; 95% confidence interval, 0.67-0.75), and allowed for correct risk estimation of up to 5 years differentiating between low- and high-risk patients.33 A study by Ramchand et al.26 reported a large single-center retrospective series of 328 patients, with 102 (31%) MACE during a mean follow-up of 3.1 years. After adjustment for medical history, the variables independently associated with outcomes were compacted myocardial thinning, elevated NT-proBNP levels, and increased LV end-systolic volumes. The combination of the 3 variables conferred a higher accuracy for risk stratification. Although these 2 approaches do not imply specific changes in clinical management, they can identify high-risk patients who might benefit from closer surveillance or low-risk patients who might not require strict follow-up. Most importantly, they are based on readily available variables that do not require complex assessment and which can therefore be easily applied in clinical practice. Future studies should elucidate whether the prospective application of such risk scores improves prognosis in this disease.
CONCLUSIONSFinal remarksLVNC is a poorly defined, heterogeneous, and controversial entity. Diagnosis is currently based on morphologic imaging variables, which have low specificity for identifying true cardiomyopathy cases. Evidence suggests that a comprehensive holistic diagnostic approach with clinical information, functional imaging variables, family screening, and genetics will more accurately differentiate physiologic hypertrabeculation from LVNC cardiomyopathy. Clinical management is also challenging due to uncertain prognosis and lack of specific guidelines. Risk stratification, using LVEF and LGE among other parameters, is recommended to identify low- and high-risk patients and to tailor follow-up.
Future directionsWe are facing a paradigm shift in cardiomyopathies due to the constantly growing knowledge of cardiovascular genetics and a deeper understanding of genotype-phenotype correlations. Therefore, we will ultimately refer to the phenotypic expression of a specific genetic variant, instead of using the terminology of traditional heart diseases. In addition, the never-ending development of cardiac imaging techniques and the application of artificial intelligence algorithms will allow for a better identification of pathologic hypertrabeculation cases, which will result in an optimization of health system resources.
FUNDINGNone.
AUTHORS’ CONTRIBUTIONSJ.F. Rodríguez-Palomares and I. Ferreira-González conceived the review, G. Casas and J.F. Rodríguez-Palomares wrote the manuscript, I. Ferreira-González critically reviewed the manuscript.
CONFLICTS OF INTERESTI. Ferreira-González has received speaker fees from Bayer, Daichii-Sankyo, Boehringer, and Sanofi, has received support for attending meetings from Bayer, Boehringer, and Sanofi, and has participated on Advisory Boards from Sanofi and Philips.
The authors thank Hannah Cowdrey for thorough revision of the manuscript for English accuracy.