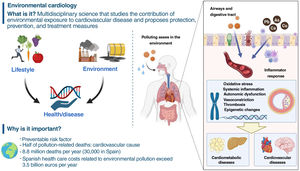
The environment is a strong determinant of cardiovascular health. Environmental cardiology studies the contribution of environmental exposures with the aim of minimizing the harmful influences of pollution and promoting cardiovascular health through specific preventive or therapeutic strategies. The present review focuses on particulate matter and metals, which are the pollutants with the strongest level of scientific evidence, and includes possible interventions. Legislation, mitigation and control of pollutants in air, water and food, as well as environmental policies for heart-healthy spaces, are key measures for cardiovascular health. Individual strategies include the chelation of divalent metals such as lead and cadmium, metals that can only be removed from the body via chelation. The TACT (Trial to Assess Chelation Therapy, NCT00044213) clinical trial demonstrated cardiovascular benefit in patients with a previous myocardial infarction, especially in those with diabetes. Currently, the TACT2 trial (NCT02733185) is replicating the TACT results in people with diabetes. Data from the United States and Argentina have also shown the potential usefulness of chelation in severe peripheral arterial disease. More research and action in environmental cardiology could substantially help to improve the prevention and treatment of cardiovascular disease.
Keywords
Cardiovascular diseases (CVD) are a leading cause of hospitalization and death in most parts of the world and develop as the result of complex interactions between genes and the environment. The undeniable gap between the incidence of CVD and the identification of risk factors has led the scientific community to investigate additional risk factors, particularly modifiable factors such as those related to the environment.1–13
In the 20th century, short- and long-term effects due to air pollution were seen to produce notable increases in cardiovascular morbidity and mortality.14–17 The systematic analysis of the increase in cardiovascular morbidity and mortality coinciding with increased air pollution in a number of US cities,18,19 as well as evidence showing a link between metals such as mercury and the risk of myocardial infarction (MI)20 or between lead and cardiovascular mortality,21 led to the concept of “environmental cardiology” in the early 2000s in an article published in Environmental Health Perspectives, an influential journal in the field of environmental health science.4
Epidemiologic studies have shown a steady increase in the risk of CVD linked to short- and long-term exposure to concentrations of polluting particulates in the environment, with the cardiovascular system most commonly affected.2,4,8,22 Several plausible physiologic and pathologic pathways have been described, for instance, increased coagulation, thrombosis, predisposition toward arrythmia, acute arterial vasoconstriction, systemic inflammatory responses, and the chronic influence of atherosclerosis.1,10,11,23–42 These effects have been linked to ischemic heart disease, congestive heart failure, MI, malignant ventricular arrhythmia, plaque vulnerability, acute thrombosis, stroke, diabetes mellitus, and hypertension.5,8,9,43–45 In this context, the largest study on the influence of the global disease burden showed that air pollution is the fourth most important risk factor after hypertension, tobacco use, and dietary factors, even ahead of hypercholesterolemia.3 Among the 6.7million deaths attributable to environmental pollution in 2019, 50% were due to CVD.3 At an individual level, people around the world would lose an average of 1.7 years of life as a result of exposure to anthropogenic air pollution, and sources not readily preventable (eg, desert dust or natural fires) are included, then the loss would rise to 2.9years.46
What is environmental cardiology?Exposure to environmental pollution—which includes chemical substances such as particulate matter with an aerodynamic diameter ≤2.5μm (PM2.5), metals and some organic compounds, and noise pollution—contributes to the risk of CVD. In the absence of a universal definition, we suggest defining environmental cardiology as the interdisciplinary science that studies the contribution of environmental exposure to CVD, with the aim of developing specific preventive or therapeutic strategies to minimize the harmful influence of environmental pollution and to promote cardiovascular health. This review focuses on PM and metals, the pollutants with the most sound scientific evidence.
CLINICAL EVIDENCEEnvironmental pollutionWhat are the effects of pollution on our health? The clinical events associated with environmental pollution have been thoroughly described in the literature.2,7 However, there does not seem to be a minimum safety threshold, and the relationship may even be supralinear, ie, the adverse effects of pollution changes at lower exposure levels are worse than when the same changes occur at higher exposure levels.47,48
Air pollution is associated with increased hospitalizations due to ischemic cardiac disease, atrial fibrillation, and heart failure2 but has also been linked with hospitalization due to aortic dissection43 and with the development of congenital heart disease.49 Some data also link air pollution to peripheral artery disease, as summarized in a recent report from the American Heart Association.50 The proinflammatory state produced by environmental pollution contributes to a poorer prognosis in CVD patients.45,51
Exposure to higher air pollution increases mortality.8,17,18,21,45,47 Although it has been traditionally presumed to result in cardiovascular mortality regardless of pollutant composition,23 recent data show that metal- and acid-rich particles are more toxic.52 The increase in daily CVD mortality seen with an increase of 10μg/m3 in the 2-day average PM2.5 was 0.55%.53 Even exposure to PM from desert dust has been associated with an increased cardiovascular mortality, both on the day of exposure and on the previous day.54
In actuality, PM is a mix of substances of varying toxicity and can include soot, hydrocarbons, sulfur and nitrogen compounds, dust, and various metals such as arsenic, cadmium, and nickel. Many pollutants form particles suspended in the air and can be inhaled. These particles may come from natural sources (forest fires, sea spray, volcanic eruptions, desert dust) or from human sources (industrial activity, transportation, heating systems, and fossil fuel combustion in general) and are classified according to size, an important factor in their harmful effects.
Particulate with a diameter between >2.5 and ≤10μm is considered coarse and is deposited in the upper airways. Fine particulate (PM2.5) is deposited in deeper areas of the lungs, from where they enter the bloodstream. They are usually produced by human activities such as woodburning, industry, construction, automobiles, and the transportation sector in general (figure 1). Other sources are forest fires, household dust, and cigarette and kitchen smoke. Ultrafine particulate (diameter ≤0.1μm [PM0.1]) is also given off by vehicles, particularly diesel-powered engines, and readily enters the systemic circulation. Fossil fuels burned for heating and cooking also generate ultrafine particulate.
Heavy metalsA growing number of epidemiologic studies, supported by experimental evidence and toxicologic studies, indicates that metal exposure increases the risk of CVD. A recent US study with over 9000 individuals showed that participants with high exposure to heavy metals (lead and cadmium) had CVD-related mortality that was 1.63-fold that of participants with low exposure.55 The Strong Heart Study,56 a cohort study with 3600 participants from populations of native Americans who had been exposed to arsenic in their drinking water, observed higher cardiovascular and all-cause mortality (hazard ratio=1.28 per increase in interquartile interval of arsenic in urine). In this study, greater exposure to cadmium was also associated with a higher risk of cardiovascular morbidity and mortality due to ischemic heart conditions, heart failure, and cerebrovascular disease.57
Environmental metals are ubiquitous, and populations are chronically exposed through food, air, tobacco smoke, and even drinking water in some areas. Therefore, the potential impact of this exposure on public health is considerable. In the United States, 32% of the decrease in cardiovascular mortality rates observed between 1988 and 2004 was explained by reduced exposure to lead and cadmium, adjusting for traditional risk factors.58
Several studies in Spanish populations have shown a relationship between metal biomarkers and various outcomes related to cardiovascular risk.9,44,59,60 The Hortega Study9,12 looked at a sample of the general population in Valladolid (Spain), finding that copper, zinc, antimony, cadmium, chromium, and vanadium concentrations were associated with the incidence of CVD over a 13-year of follow-up. In the Aragon workers’ health study (AWHS cohort),61 workers with urine metal concentrations similar to those seen in the study by Hortega et al., high urine concentrations of inorganic arsenic, cadmium, titanium, and perhaps antimony were linked with varying degrees of subclinical atherosclerosis.41
The AWHS cohort study is particularly interesting, given that earlier studies usually focused only on the carotid arteries rather than vascular territories such as the femoral and coronary territories. In a Bangladeshi study62 and in the Strong Heart Study,63 arsenic exposure has been associated with an increased thickness of the intima-media layers of the carotid. A direct association has also been found between blood cadmium concentrations and the thickness of the intima-media layers of the carotid of Austrian women,11 and the prevalence of atherosclerotic plaque in a Swedish study.10,42 In the AWHS study, arsenic and cadmium were linked to the presence of carotid plaque, but cadmium and titanium were also linked to femoral plaque, and titanium and possibly cadmium and antimony were linked to coronary calcium. These associations persisted after adjusting for other metals and for classic risk factors.61
Metal levels could aid early screening for individuals at risk due to exposure, thus allowing individualized measures to be taken before clinical events occur. A randomized clinical trial evaluating the administration of a heavy-metal chelator agent known as disodium ethylenediaminetetraacetic acid (EDTA) vs placebo in patients with a prior MI showed an improvement in the combined primary outcome (time to all-cause death, recurrent MI, coronary revascularization, hospitalization due to angina or stroke) in the intervention group (hazard ratio=0.82).64 The subgroup of diabetic patients had even greater benefits and has a risk reduction of 41%.65
EVIDENCE FROM MECHANISTIC STUDIESAir pollution enters the body through the alveoli and promotes the development of CVD through the activation of various mechanisms, such as inflammation, endothelial dysfunction, oxidative stress, autonomic dysfunction, and thrombogenicity.8 Other pollutants can enter the gastrointestinal system through water or other drinks.
Increased inflammation is associated with ischemic events, arrythmia, heart failure, and a lack of control of cardiovascular risk factors. Air pollution promotes the production of interleukin 6 (IL-6) and C-reactive protein, inflammatory markers associated with a higher risk of CVD.24 Exposure to greater environmental pollution in the 24hours prior to hospitalization modulates the inflammatory profile of patients with MI. Greater acute exposure to sulfur dioxide, a gas linked to fossil fuel combustion and industrial activity, has been associated with larger MIs and stronger white blood cell activity.25
Chronic exposure to high air pollution has been associated with the formation and vulnerability of coronary plaque at values far below European Union limits (annual PM2.5 <25μg/m3). Optical coherence tomography showed a higher prevalence of thin-cap fibroatheroma and macrophagic infiltrates in patients with acute coronary syndrome exposed to a higher annual PM2.5.26 Even within the exposure limits recommended by the World Health Organization (WHO) before 2021 (annual PM2.5 <10μg/m3, currently annual PM2.5 <5μg/m3), the highest levels are associated with great arterial white blood cell activity and leukopoietic activity measured by 18F-FDG uptake, activity levels associated with cardiovascular events during patient follow-up.27
Oxidative stress also plays a key role in the pathogenic vascular and myocardial effects of environmental pollution.28 Epidemiologic and controlled-exposure studies have observed positive associations between PM2.5 concentrations and concentrations of several plasma and urine biomarkers for oxidative stress,29 including increases in thiobarbituric acid reactive substances (TBARS), a marker of lipid peroxidation.30 The role of oxidative stress in endothelial damage is supported by data from animal models. Controlled, short-term exposure to diesel exhaust at concentrations similar to those detected in urban environments produces transitory microcirculation dysfunction.32 The inhalation of diesel particulate causes endothelial dysfunction in rats, an effect that can be reverted using a treatment with oxygen free radical scavengers.33 Oxidative stress also has a prominent role on the myocardium. Isolated rat cardiomyocytes exposed to diesel particulate exhibited lower contractility, which was attenuated with antioxidants.34 In another rodent experiment, exposure to diesel particulate increased susceptibility to myocardial damage induced by ischemia-reperfusion, an effect associated with the local generation of oxygen free radicals and proinflammatory cytokines.35 In rats, prolonged PM2.5 exposure causes damage to myocardial cells with ultrastructural and inflammatory infiltrate changes36 and mitochondrial abnormalities,37 leading to remodeling and hypertrophy.38
Several environmental pollutants (eg, lead) can cause autonomic dysfunction and can trigger reflex arcs that affect heart rate and promote arrhythmia.39 Most epidemiologic studies have described adverse associations between various indices of heart rate variability and the concentrations of PM2.5 and other pollutants,40 which may be related to the higher incidence of arrythmia reported in patients with MI.45 Additionally, calcium/calmodulin-dependent protein kinase II (CaMKII) has been possibly implicated in these proarrhythmic effects.66 These mechanisms are also involved in the vascular toxicity of metals such as cadmium and lead.67 For instance, lead can substitute for calcium in calmodulin. This mechanism has been linked to the synthase regulation of nitric oxide, affecting the production of nitric oxide, key for endothelial function and for the inhibition of platelet aggregation.68
Both PM and the gaseous components of air pollution also favor the appearance of thrombi.69 Available evidence indicates that acute exposure to PM2.5 shifts the hemostatic balance toward a prothrombotic state. This state has been associated with elevated oxidative stress and inflammation biomarkers, as well as with platelet activation and fibrinolysis reductions.69 Furthermore, exposure to ultrafine particulate increases the in vitro formation of factor XIIa, whereas postexposure thrombin formation is inhibited in animals deficient in this factor, indicating direct modulation of the intrinsic coagulation pathway.70
Numerous studies have linked metal exposure with biomarkers of oxidative stress67,71 and metabolic72 and epigenetic patterns.73,74 Gene-environment interactions are another field of growing interest, as they could aid early screening for individuals who may benefit from stronger prevention strategies and could identify biological mechanisms to help understand the role of the environment in CVD and broaden the strategies used to prevent and monitor these diseases.
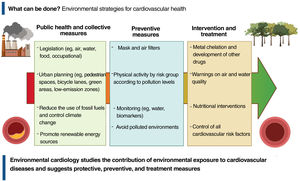
PREVENTION STRATEGIES AND TREATMENTFirst of all, what can be done? Public health measures, through legislation and mitigation measures and control of environmental pollutants, are key strategies to minimize air and water pollution as well as food contamination with toxic compounds and to protect populations from the harmful effects of these substances on cardiovascular health (figure 2). To encourage such measures, in September 2021 the WHO published new air quality guidelines, recommending that countries establish a PM2.5 annual limit of 5μg/m3 and a daily limit of 15μg/m3. These guidelines are well below the legally established limits in the European Union, the United States, and most other countries. In Spain and many Latin American countries, most cities have pollution levels far above the WHO guidelines and even above the limits established by the countries themselves. As a collective strategy, therefore, legislation should be proposed and enacted to comply with WHO recommendations. Environmental policies that reduce pollution levels around the world are, for instance, the optimal design of bicycle lanes and pedestrian spaces, the promotion of public transportation, measures to reduce emissions from fuel and other toxic gases, and new building codes for sustainable housing and offices, the prohibition and elimination of old, highly-polluting heating systems, and the promotion of green areas.
Individual protection strategies (figure 2) could include respiratory barriers as an option for outdoor environments. Nevertheless, respiratory protection such as gauze, cotton, surgical, or cloth masks has not yet been validated for reducing exposure to PM2.5, hence they are not recommended in this regard. Other types of personal protective equipment, such as face masks with respirators (eg, N95masks), are specifically designed and validated to filter 95% of particles, including PM2.5. However, no personalized intervention intended to reduce pollution exposure has been shown to reduce cardiovascular events.22 More studies would be needed to assess the effectiveness of these kinds of personal barriers.
Indoors, PM2.5 concentrations can be reduced with high-efficiency air purification systems in home air conditioning installations. Although these systems has been reported to have an impact in lowering inflammatory and circulatory thrombogenic biomarkers and blood pressure,75 the evidence is insufficient, discouraging statements on the cardiovascular benefits of air purifiers.76 Additionally, real-time information on air pollution levels could be a way to protect public health under certain circumstances.77 However, there is not enough evidence on the clinical impact of this measure and on who may benefit.
Diet may also have an impact on the effect of environmental pollution on our health. Dietary supplements with omega-3 fatty acids have been associated with short-term subclinical cardiovascular benefits against PM2.5 exposure,78 and supplements with vitamin B (folic acid, B6, B12) have shown a possible benefit in mitigating the effects of PM2.5 on inflammation and autonomic cardiac dysfunction in pilot studies.79 Folic acid supplements have also shown an ability to eliminate arsenic from the body more quickly and to reduce its toxicity.80
Regarding physical exercise in areas with high environmental pollution, the interaction depends on multiple mechanisms. Public health models have estimated that in most cases, the benefits of physical exercise outweigh the risks from pollution,81 although evidence is insufficient for people with established risk factors of CVD.82
Research on treatments has focused on eliminating metals from the body. Cadmium and lead, which are divalent cations, can be treated with high-affinity chelators, such as EDTA and its salts (disodium EDTA and calcium disodium EDTA). To date, several studies83–85 have shown that intravenous disodium EDTA enhances the urinary excretion of toxic metals, including cadmium and lead. Arenas et al.83 published the results of disodium EDTA infusion in patients with a history of MI, which produced a 71% increase in total urinary excretion of metals compared with original levels, with a notable effect on lead (3.835% increase) and cadmium (633% increase). High excretion of these metals after EDTA administration is indicative of the cumulative exposure to these metals over the years from various sources (air pollution, water, tobacco, soil and food contamination, etc.) and of the difficulty in eliminating these toxic metals, highly similar to essential metals such as calcium and zinc, which they replace in many proteins and enzymes.
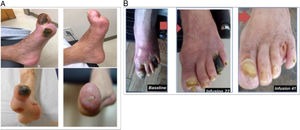
The Trial to Assess Chelation Therapy (TACT) is a double-blind, placebo-controlled, 2×2 factorial clinical trial to evaluate the risks and benefits of 40 disodium EDTA infusions compared with placebo in 1708 participants with a history of MI. Patients received follow-up for 5 years. The trial showed a significant reduction of 18% (P=.035) of the composite main outcome of death, MI, stroke, coronary revascularization, and hospitalization for angina.64 The most obvious benefit was seen in patients with a history of diabetes, with a 41% reduction in the hazard ratio of the combined cardiovascular endpoint (P <.001) and a 43% reduction in all-cause mortality (P=.011).65 At present, the TACT2 clinical trial (NCT02733185) is reproducing the TACT results in people with diabetes. The results from TACT2 will be available by late 2023. In a recent meta-analysis, the patients most clearly benefitting from EDTA treatment were those with diabetes and peripheral artery disease.86 Indeed, several cases have been published showing remarkable benefits in patients with severe peripheral artery disease in the United States87 and Argentina88 (figure 3). In the future, metal chelators that can be taken orally or are easier to administer may be developed. For instance, an oral chelator, known as succimer or dimercaptosuccinic acid (DMSA) and mainly used to treat saturnism (lead poisoning), is now available but has still not been tested for potential cardiovascular benefits.
Photographs of patients with severe peripheral artery disease at the beginning and after repeated infusions of disodium EDTA (ethylenediaminetetraacetic acid) chelator in a patient in Miami, United States (A, reproduced with permission from Arenas et al.87) and Rosario, Argentina (B, reproduced with permission from Ujueta et al.88).
A common question is whether it might be useful to measure metals in blood or urine to identify individuals at risk of CVD and to apply early intervention, and to determine the concentrations that could be considered toxic. There are several well-established metal biomarkers, with half-lives and sample types (blood, urine, others) considered most suitable for each metal (table 1). However, additional studies are needed to determine whether routine concentration testing could be helpful in clinical practice.
Metal biomarkers associated with cardiovascular disease.
| Metal | Sample | Half-life | Method | Additional Information | Possible Reference Range* |
|---|---|---|---|---|---|
| Arsenic | Urine | 1 to 30 d depending on species | Liquid chromatography–mass spectrometry for species separation | Avoid eating fish on the days prior to collecting the sample | 10μg/L (first morning urine) |
| Cadmium | BloodUrine | 30 to 100 dDecades | Mass spectrometry (blood and urine) | Smokers have high values | 1μg/L1μg/L (first morning urine) |
| Lead | BloodBone | 30 to 100 dDecades | Mass spectrometry (blood)X-ray fluorescence (bone) | Blood is the usual marker, whereas bone is used for research purposes | 3.5μg/dL (blood) |
For arsenic, urine concentration according to correspondence with the water limit; for cadmium, values around 3-fold the geometric mean in blood and urine from the National Health and Nutrition Examination Survey (NHANES),55 clearly related to the toxicity level; blood lead concentration is based on the guidance from the Centers for Disease Control and Prevention for children and pregnant women, figures clearly associated with cardiovascular disease.
The residual risk is supported by ever-increasing evidence and probably includes uncontrolled risk factors as well as risk factors not considered or not yet known. Environmental pollution, although one of the cardiovascular risk factors,3,11,12,46 has not yet achieved widespread health care and social awareness compared with other risk factors. Training in this field is greatly lacking in cardiology and, therefore, the effect of environment should be taught in training curricula, eg, as part of the curriculum on climate change and environmental health.
For the first time, the 2021 European Prevention Guidelines include a specific section on environmental pollution and classify air pollution reduction as class I, recommending lowering PM emissions and gaseous pollutants, reducing fossil fuel use, and limiting carbon dioxide emissions as measures to lower morbidity and mortality due to CVD.89 Consequently, the European Commission agreed on a series of measures to be implemented by 2030 to reduce harmful emissions from traffic, power plants, and agriculture, within the context of fighting climate change.
Patients at risk for CVD should be advised to avoid long-term exposure to areas with high environmental pollution. Opportunistic risk screening programs (class IIb recommendation, level of evidence C) may be considered.89 In addition, according to the same guidelines, patient and health care professional organizations are essential for training and political initiatives. “Clean air” legislation should be strengthened to encourage lower particulate emissions and the use of public transportation. Learning about the impact of environmental pollution should start in schools and families. Patient training (eg, patient schools) could also be fostered.
Last, environmental pollution also has a financial impact. Air pollution-related mortality represents a cost to the global economy of around US$225 billion dollars in lost wages and over US$5 trillion in welfare losses.90 Problems derived from air pollution cost every person living in Spain nearly 1000 euros each year.91
FUNDINGThe authors declare the following sources of funding: A. Navas-Acién from the National Institutes of Health (P42ES033719, P30ES009089); J. Bañeras, B. Benito, and A. Rodríguez Sinovas from the Instituto de Salud Carlos III (ISCIII) (PI20/01649 and CIBERCV), co-funded by the European Regional Development Fund (ERDF-FEDER, a way to build Europe); A. Domínguez Rodríguez and N. Báez-Ferrer of the Instituto de Salud Carlos III (ISCIII) (PI21/00404), co-funded by the European Regional Development Fund (ERDF-FEDER, a way to build Europe). J. Iglesies-Grau, M. Téllez-Plaza, V. Arrarte, R. Campuzano Ruiz, A. Cecconi, Francisco Ujueta, C. Vozzi, and G.A. Lama have no sources of funding related to this article.
AUTHORS’ CONTRIBUTIONSAll authors contributed to the writing and critical review of this article.
CONFLICTS OF INTERESTNone declared.