Left ventricular torsion decreases during transmural myocardial ischemia, but the effect of exercise on left ventricular torsion has not been widely studied. We hypothesized that exercise-induced ischemia may impair left ventricular torsion. Therefore, our aim was to study the effects of exercise on left ventricular torsion in patients with an ischemic response to exercise echocardiography and in patients with a normal response.
MethodsA retrospective analysis was performed in 172 patients with ejection fraction ≥ 50% who were referred for exercise-echocardiography and studied by speckle imaging at rest, peak and postexercise. Torsion was defined as apical rotation – basal rotation (in degrees) / left ventricular length (in centimeters). A total of 114 patients had a normal exercise echocardiography and 58 patients had an ischemic response to exercise echocardiography.
ResultsPatients with ischemic response to the test exhibited less basal rotation at peak exercise (+0.30° [2.39°] vs –0.65° [2.61°] in the normal group; P = .03), whereas peak apical rotation was similar (ischemic response to the test, 7.80° [3.51°]; normal response, 7.27° [3.28°]; P =.36). Torsion at peak exercise was also similar (1.07° [0.60°] in the ischemic response to the test group vs 1.16° [0.57°] in normal group; P =.37). A more impaired peak basal rotation was found in patients with anterior or anterior+posterior involvement (anterior ischemic response, +1.22° [2.45°]; anterior + posterior ischemic response, –0.20° [2.25°]; posterior ischemic response, –0.71° [1.96°]; normal response, –0.65° [2.60°]; P =.02).
ConclusionsBasal rotation at peak exercise is impaired in patients with an ischemic response to exercise echocardiography, particularly in those with anterior involvement. Apical rotation and torsion are similar to those in patients with normal exercise echocardiography.
Keywords
Although left ventricular torsion (LV-Tor) has been studied at rest in several heart diseases,1–3 including coronary heart disease,4–6 there are no data in patients with coronary ar-tery disease (CAD) under ischemic conditions unmasked by exercise. This deformation has been found to be increased with inotropic stimulation with dobutamine and decreased during transmural myocardial ischemia.7 However, the effect of ischemia induced by exercise on human torsion might be different, because exercise-induced ischemia may be limited to the endocardium. Recently, speckle tracking imaging analysis has shown promising results in the assessment of LV-Tor.7,8 We aimed to study the effects of exercise on LV-Tor in patients with an ischemic response to exercise echocardiography (IR-ExE) and in those with a normal response.
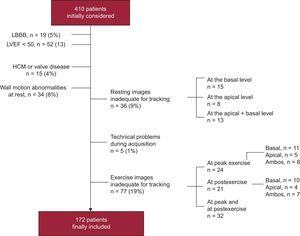
METHODSPatientsWe assessed a series of 410 consecutive patients referred for ExE to our center for clinical reasons. Patients with left bundle branch block, left ventricular (LV) systolic dysfunction as defined by a left ventricular ejection fraction (LVEF) < 50%, clinical diagnosis of hypertrophic cardiomyopathy or evidence of significant valvular disease, and patients with resting wall motion abnormalities were excluded. Patients with unfeasible resting or exercise studies for assessment of LV-Tor were also excluded. Figure 1 depicts the number and percentage of patients excluded for the different reasons. No patients were excluded due to suboptimal acquisition of 2-dimensional images, which could have rendered the study nondiagnostic. The final retrospectively studied population included 172 patients who were divided into 2 groups: the normal group was composed of 114 patients with normal ExE results, and the ischemic group of 58 patients with an IR-ExE. A total of 21 patients (12%) had a history of myocardial infarction but their resting echocardiogram was completely normal.
Coronary angiography was performed at the discretion of the responsible team (within 6 months). Angiogram analyses were performed by the angiographic laboratory team using a qualitative approach. The invasive cardiologists were not blinded to the ExE results, although they were blinded to the LV-Tor measurements. Patients gave written informed consent before the tests.
Exercise EchocardiographyExE was performed on a treadmill according to different protocols (Bruce 77%, other protocols 23%). Heart rate, blood pressure, and a 12-lead electrocardiogram were obtained at baseline and at each stage of the exercise protocol. Exercise end-points included physical exhaustion, significant arrhythmia, severe hypertension (systolic blood pressure > 240mmHg or diastolic blood pressure > 110mmHg), severe angina, and severe hypotensive response (decrease > 20mmHg in systolic blood pressure). Ischemic electrocardiogram abnormalities during the test were defined as the development of ST segment deviation ≥ 1mm 80ms after the J point.
Echocardiography was performed from the apical long-axis, 4- and 2-chamber views, and the parasternal long- and short-axis views at the apical level and at the basal level, at rest and at peak exercise.9,10 Images at rest were taken in the lateral decubitus position, at peak exercise with the patient still exercising on the treadmill. Images at peak exercise were used for the diagnosis of ischemia. At postexercise echocardiography (within 40 s), with the patient again lying in lateral decubitus position, the short-axis views at the apical level and at the basal level were again recorded for assessment of LV-Tor at postexercise. At least 3 cardiac cycles were obtained for the assessment of torsion at peak and at postexercise in the short-axis basal and apical views. Therefore, to detect ischemia, we relied on peak exercise imaging, whereas LV-Tor was measured at peak and at postexercise and both values were given. Regional wall motion was evaluated for ischemia assessment with a 16-segment model of the LV.11 Each segment was graded on a 4-point scale, with normal wall-motion scoring = 1, hypokinetic = 2, akinetic = 3, and dyskinetic = 4. The wall motion score index was calculated at rest and at peak exercise as the sum of the scores divided by the number of segments. Ischemia was defined as the development of new wall motion abnormalities with exercise, with the exception of isolated hypokinesia of the inferobasal segment.12 Left anterior descending (LAD) coronary artery ischemia was considered when there were new wall motion abnormalities in the septal, apical and/or anterior segments; right coronary artery/left circumflex artery ischemia was considered when there were new wall motion abnormalities in other segments of the LV. Patients with ischemia in the LAD territory were grouped as having anterior circulation territory involvement, those with ischemia in the right coronary artery or left circumflex artery territories as having posterior circulation involvement, and those with ischemia in the LAD and the right coronary artery or left circumflex artery territories as having anterior + posterior circulation territory involvement. The ratios of early transmitral flow and early diastolic flow wave velocities at the mitral annulus (E/e¿) were also assessed at rest and after exercise. In case of merging, they were measured once they were separated. Beta-blocking agents were withdrawn for the ExE in most patients.
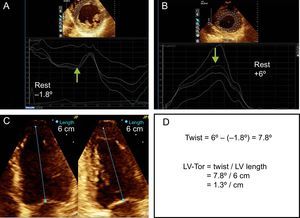
Torsion AssessmentThe short axis views at the apical and at the basal levels were used for assessment of LV-Tor by speckle tracking using commercially available technology (Philips ie33; Andover, United States; speckle tracking package software, QLab version 8.1.2). For this purpose, the short axis basal view should include the mitral valve and the short axis apical view should show a spherical shape of the myocardium with no papillary muscle. Images were obtained at a frame rate of 70-100 fps. The stored images were analyzed at least 30 days after the test to avoid bias due to knowledge of the ExE results. Maximal counterclockwise rotation (in degrees) was measured from the apical short axis view and counted as a positive value, whereas maximal clockwise rotation (in degrees) was measured from the basal short axis view and counted as a negative value. The region of interest included the entire myocardial thickness. Care was taken to avoid the pericardium within the sample size. The adequacy of tracking was verified manually with readjustment of the region of interest if necessary. Twist (in degrees) was defined as apical rotation – basal rotation, and LV-Tor as Twist (in degrees) / LV length (in centimeters).13 LV length was obtained from the mean of the values of LV length in the apical 4- and 2-chamber views at rest and at peak exercise (Figure 2). Time (in milliseconds) to maximal apical and basal rotation at rest and at peak exercise were also measured and the values corrected by the RR interval. The untwist of the apical rotation was measured at 25% of the untwisting period at rest and at peak exercise14 and the values given as a percentage as: 100 – (apical rotation at 25% of the untwisting interval/maximal apical rotation). Finally postexercise imaging was also analyzed and the values of apical and basal rotation, twist and LV-Tor were calculated. At rest and at peak exercise we measured global, endocardial and subepicardial rotation at the basal and at the apical level. At postexercise, we measured global, endocardial and subepicardial basal rotation, whereas global values are given for apical rotation. LV-Tor measurements in the ischemic and normal groups were compared. All measurements were performed by board certified cardiologists.
Measurement VariabilityTorsion measurements were performed by a single observer with experience of about 200 speckle tracking recordings during ExE. To assess reproducibility, the same measurements were repeated by the same observer on the same images in 22 randomly chosen studies, at least 4 weeks apart. These same studies were also measured by a second observer blinded to the results of the other observer to determine interobserver variability.
ObjectivesOur goal was to study the effects of exercise on LV-Tor in patients with an IR-ExE and in those with a normal result. Speckle tracking was used to measure basal and apical rotations at rest and at exercise. Twist and LV-Tor were derived from these assessments.
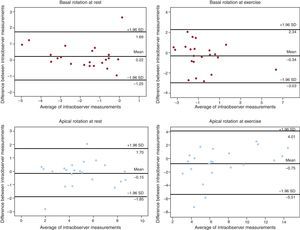
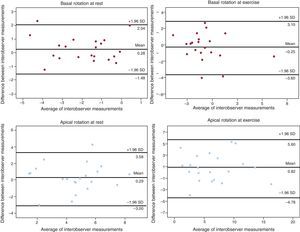
Statistical AnalysisCategorical variables are reported as percentages and comparison between groups based on the chi-square test. Continuous variables are reported as mean (1 standard deviation) for those following a normal distribution and as the median [interquartile range] values for those following a nonnormal distribution. Normality was assessed by the Kolmogorov-Smirnov test. Intergroup differences were assessed with the unpaired Student t test or the Mann-Whitney U test, as appropriate. The ANOVA (analysis of variance) test was used for analysis of differences among more than 2 groups. A P value of <.05 was considered statistically significant. Pearson's correlation coefficient (r) was used to describe the relationship between continuous variables. We explored the correlations between the main LV-Tor parameters (LV-Tor, apical and basal rotation) and age, exercise workload, E/e¿ ratios, LVEF and peak wall motion score index. PIN and POUT were set at 0.05 and 0.10, respectively. Variables of interest that correlated with the dependent variable on univariate analysis were included in a stepwise linear regression analysis. Multivariate associations of IR-ExE were also assessed after the variables significantly associated in the univariate analysis were entered in a logistic regression analysis model. Statistical analyses were performed using SPSS software, version 15.0 (SPSS Inc.; Chicago, Illinois, United States). Inter- and intraobserver variability in the assessment of torsion were assessed by Bland-Altman graphics and interclass correlation coefficients. An interclass correlation coefficient ≥ 0.82 is considered as moderate agreement.
RESULTSClinical Characteristics and Angiographic ResultsTable 1 summarizes the clinical and ExE characteristics in patients with and without IR-ExE. As expected, patients with IR-ExE had a worse clinical profile with lower functional capacity and a higher wall motion score index at exercise than patients with normal ExE. Among the 58 patients with IR-ExE, 28 had multiterritory involvement (48%), and 30 had single-territory IR-ExE (52%). Ischemic response in the anterior circulation territory was observed in 23 patients, in the posterior circulation territory in 9, and in both territories in 26 patients.
Clinical, Resting and Exercise Echocardiographic Characteristics in the 2 Patient Groups
| Variable | Normal (n = 114) | IR-ExE (n = 58) | P value |
| Age, mean (SD), y | 60 (13) | 68 (9) | <.001 |
| Male sex | 57 (50) | 44 (72) | .007 |
| Diabetes mellitus | 23 (20) | 12 (21) | .91 |
| Hypertension | 65 (57) | 40 (69) | .15 |
| Left ventricular hypertrophy | 55 (48) | 30 (52) | .45 |
| Previous myocardial infarction | 14 (12) | 7 (12) | .98 |
| Previous revascularizations | 19 (17) | 18 (31) | .06 |
| Beta-blockers at the time of the ExE | 15 (13) | 9 (15) | .63 |
| Resting heart rate, mean (SD), bpm | 79 (15) | 73 (12) | .01 |
| Peak heart rate, mean (SD), bpm | 149 (19) | 141 (20) | .01 |
| Resting systolic blood pressure, mmHg | 120 [120-140] | 130 [120-140] | .13 |
| Peak systolic blood pressure, mean (SD), mmHg | 157 (23) | 165 (23) | .06 |
| Angina during ExE | 21 (18) | 19 (33) | .047 |
| Positive electrocardiogram | 17 (15) | 27 (46) | <.001 |
| Exercise workload, mean (SD), METs | 10.3 (4.0) | 8.9 (2.5) | .007 |
| Resting WMSI | 1 [1-1] | 1 [1-1] | – |
| Peak WMSI | 1.00 [1.00-1.00] | 1.25 [1.13-1.50] | <.001 |
| Resting LVEF, mean (SD), % | 67 (8) | 65 (8) | .29 |
| Peak LVEF. mean (SD), % | 70 (10) | 61 (12) | <.001 |
| E/e’ at rest | 10.5 [8.0-13.8] | 12.1 [9.6-14] | .11 |
| E/e’ at postexercise postexercise* | 8.3 [7.1-10.0] | 8.3 [7.3-9.7] | .24 |
E/e¿, ratios of early transmitral flow and early diastolic flow wave velocities at the mitral annulus; ExE, exercise echocardiography; IR-ExE, ischemic response to exercise echocardiography; LVEF, left ventricular ejection fraction; METs, metabolic equivalents; SD, standard deviation; WMSI, wall motion score index.
Data are expressed as no. (%), mean (standard deviation) or median [interquartile range].
Coronary angiographies were performed mainly in patients with abnormal ExE results: 35 of the 58 patients in the IR-ExE group, and 14 of the 114 patients in the normal ExE group. Twenty-five of the 35 patients in the IR-ExE group had significant stenosis (> 50% luminal narrowing), as did 5 of the 14 patients in the group with normal ExE results. Among the 30 patients with CAD on angiography, 13 had multivessel disease, and 17 had single-vessel disease involving the LAD coronary artery in 14 patients and the left circumflex artery coronary artery in 3 patients. Multivessel CAD was present in 12 of the 35 patients with IR-ExE who underwent angiography (34%) and in only 1 of the 14 without IR-ExE who underwent angiography (7%).
Left Ventricular TorsionLV-Tor parameters at rest were similar between patients with and without a history of CAD, based on previous infarction or revascularization procedures (basal rotation, –1.23° [2.30°] vs –0.81° [1.97°]; apical rotation, 5.10° [2.42°] vs 4.97° [2.25°]; twist, 6.32° [2.93°] vs 5.78° [2.58°]; LV-Tor, 0.87° [0.42°] vs 0.84° [0.40°], respectively, all P = not significant).
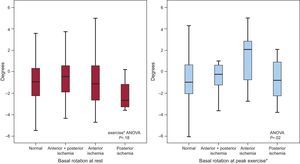
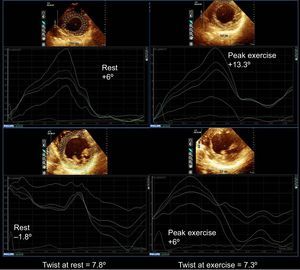
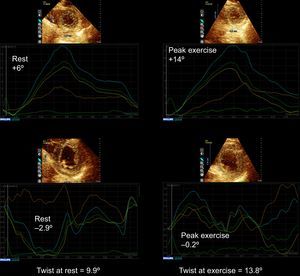
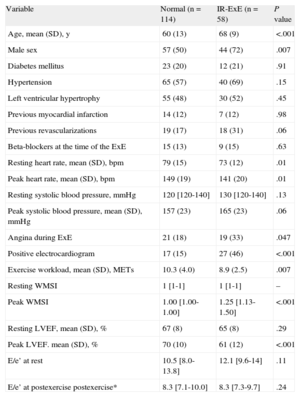
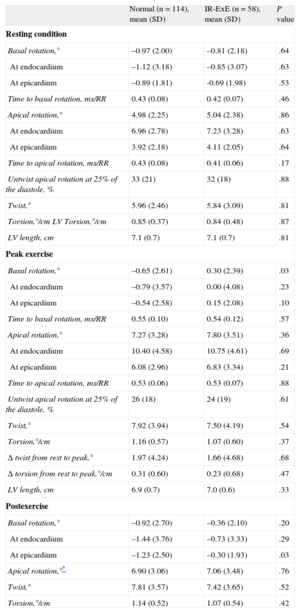
Table 2 shows the measurements of torsion at rest and at exercise in patients with normal ExE and in patients with IR-ExE. Measurements were similar at rest. At exercise, patients with IR-ExE exhibited less magnitude of the clockwise basal rotation. Global apical rotation values were not statistically different in patients with and without IR-ExE. The resulting twist and torsion were similar between groups. The same results were reproduced at postexercise imaging. Torsion measurements at rest were also not statistically different in patients with IR-ExE in the anterior, posterior, or both circulation territories. However, at exercise, the magnitude of the basal rotation was lower in patients with ischemic response involving the anterior circulation territory (Figure 3). Figure 4 (and supplementary material videos 1 and 2) is an example of a patient with global ischemic response showing blunted basal rotation and increased apical rotation from rest to exercise, leading to decreased twist with exercise. Figure 5 corresponds to a patient with ischemic response in the LAD and right coronary artery territories in whom increased apical rotation with minor changes in basal rotation led to increased twist.
Torsion Measurements at Rest, Peak and Postexercise in the 2 Patient Groups
| Normal (n = 114), mean (SD) | IR-ExE (n = 58), mean (SD) | P value | |
| Resting condition | |||
| Basal rotation,° | –0.97 (2.00) | –0.81 (2.18) | .64 |
| At endocardium | –1.12 (3.18) | –0.85 (3.07) | .63 |
| At epicardium | –0.89 (1.81) | -0.69 (1.98) | .53 |
| Time to basal rotation, ms/RR | 0.43 (0.08) | 0.42 (0.07) | .46 |
| Apical rotation,° | 4.98 (2.25) | 5.04 (2.38) | .86 |
| At endocardium | 6.96 (2.78) | 7.23 (3.28) | .63 |
| At epicardium | 3.92 (2.18) | 4.11 (2.05) | .64 |
| Time to apical rotation, ms/RR | 0.43 (0.08) | 0.41 (0.06) | .17 |
| Untwist apical rotation at 25% of the diastole, % | 33 (21) | 32 (18) | .88 |
| Twist,° | 5.96 (2.46) | 5.84 (3.09) | .81 |
| Torsion,°/cm LV Torsion,°/cm | 0.85 (0.37) | 0.84 (0.48) | .87 |
| LV length, cm | 7.1 (0.7) | 7.1 (0.7) | .81 |
| Peak exercise | |||
| Basal rotation,° | –0.65 (2.61) | 0.30 (2.39) | .03 |
| At endocardium | –0.79 (3.57) | 0.00 (4.08) | .23 |
| At epicardium | –0.54 (2.58) | 0.15 (2.08) | .10 |
| Time to basal rotation, ms/RR | 0.55 (0.10) | 0.54 (0.12) | .57 |
| Apical rotation,° | 7.27 (3.28) | 7.80 (3.51) | .36 |
| At endocardium | 10.40 (4.58) | 10.75 (4.61) | .69 |
| At epicardium | 6.08 (2.96) | 6.83 (3.34) | .21 |
| Time to apical rotation, ms/RR | 0.53 (0.06) | 0.53 (0.07) | .88 |
| Untwist apical rotation at 25% of the diastole, % | 26 (18) | 24 (19) | .61 |
| Twist,° | 7.92 (3.94) | 7.50 (4.19) | .54 |
| Torsion,°/cm | 1.16 (0.57) | 1.07 (0.60) | .37 |
| Δ twist from rest to peak,° | 1.97 (4.24) | 1.66 (4.68) | .68 |
| Δ torsion from rest to peak,°/cm | 0.31 (0.60) | 0.23 (0.68) | .47 |
| LV length, cm | 6.9 (0.7) | 7.0 (0.6) | .33 |
| Postexercise | |||
| Basal rotation,° | –0.92 (2.70) | –0.36 (2.10) | .20 |
| At endocardium | –1.44 (3.76) | –0.73 (3.33) | .29 |
| At epicardium | –1.23 (2.50) | –0.30 (1.93) | .03 |
| Apical rotation,°* | 6.90 (3.06) | 7.06 (3.48) | .76 |
| Twist,° | 7.81 (3.57) | 7.42 (3.65) | .52 |
| Torsion,°/cm | 1.14 (0.52) | 1.07 (0.54) | .42 |
IR-ExE, ischemic response to exercise echocardiography; LV, left ventricular; SD, standard deviation.
Basal rotation at rest and at peak exercise in patients with normal exercise echocardiography, and in patients with ischemic response to exercise echocardiography in the posterior, anterior or anterior+posterior coronary distribution territories. The median [interquartile range] values are shown. ANOVA, analysis of variance: NS, not significant. *Basal rotation at peak exercise in: normal vs anterior ischemia, P = .003; anterior + posterior vs. anterior ischemia, P = .05; posterior vs anterior ischemia, P = .06; other intergroups comparisons, P = NS.
Example of a patient with a global ischemic response to exercise echocardiography showing impairment of basal rotation and improvement of apical rotation from rest to peak exercise, resulting in decreased twist with exercise (supplementary material videos 1 to 2). On the top, apical rotation at rest and at peak exercise, on the bottom basal rotation at rest and at peak exercise. Multivessel coronary artery disease was demonstrated on angiography.
Example of a patient with apical and posterobasal ischemic response to exercise echocardiography. Although basal rotation decreases during exercise, twist increases due to the increase in apical rotation with exercise. On the top, apical rotation at rest and at peak exercise, on the bottom basal rotation at rest and peak exercise. Multivessel coronary artery disease was demonstrated on angiography.
LV-Tor at rest correlated weakly with measurements of LV systolic function (LVEF at rest, r = 0.19; P = .02), and with age (r = 0.19; P = .02) and did not correlated with E/e¿ at rest. In the multivariate analysis, both variables achieved statistical significance (age, P = .04; LVEF, P = .048; r2 = 0.06). Apical rotation at rest also correlated with resting LVEF (r = 0.28; P < .001) and with age (r = 0.20; P =.01). Both variables were also associated with apical rotation in the multivariate analysis (age, P = .04; LVEF, P = .002; r2 = 0.10).
LV-Tor at peak exercise only correlated with age (r = 0.25; P = .002), whereas apical rotation at peak exercise correlated with age (r = 0.31; P < .001) and with achieved METs (metabolic equivalents) (r = −0.20; P = .01). In the multivariate analysis only age remained a predictor of peak apical rotation (P < .001; r2 = 0.10). Basal rotation at peak exercise only correlated significantly with achieved METs (r = −0.25; P = .002). Not a single LV-Tor parameter at peak exercise (global LV-Tor, apical or basal rotations) was found to be significantly correlated with the ischemic burden (peak wall motion score index, peak LVEF).
Multivariate associates of an IR-ExE were only age (P = .009; odds ratio [OR] = 1.07; 95% confidence interval [95%CI], 1.02-1.13) male sex (P = .004; OR = 3.93; 95%CI, 1.57-9.87), and electrocardiogram positivity during ExE (P = .005; OR = 3.66; 95%CI,1.48-9.03). LV-Tor parameters were not independently related to an IR-ExE after adjustment. Variables entered in the model were those from Tables 1 and 2 that were significantly different between groups.
Variability in the Assessment of Left Ventricular TorsionBland-Altman analysis showed acceptable results for intra- and interobserver variability (Figures 6 and 7). Intraclass correlation coefficients between the 2 assessments for apical rotation at rest, apical rotation at exercise, basal rotation at rest, and basal rotation at exercise were 0.95 (95%CI, 0.89-0.98), 0.87 (95%CI, 0.70-0.95), 0.93 (95%CI, 0.84-0.97), and 0.88 (95%CI, 0.72-0.95), respectively, when performed by the same observer, and 0.80 (95%CI, 0.52-0.92), 0.91 (95%CI, 0.77-0.96), 0.90 (95%CI, 0.76-0.96), and 0.82 (95%CI, 0.57-0.93), when performed by 2 observers.
To our knowledge, this is the first study to assess LV-Tor during exercise on a treadmill, instead of using a bicycle or stimulation with dobutamine. In agreement with some of these studies, assessment of LV-Tor is unfeasible in a significant percentage of patients during stress.15 The reasons for unfeasible images for speckle tracking at exercise include tangential short axis basal views. In contrast, it was easier to acquire feasible short axis apical views during exercise.
The main finding was that an ischemic response to exercise echocardiography led to a blunted clockwise rotation at the basal level (more positive values), but similar twist and LV-Tor in comparison with patients without ischemia. A lower magnitude of the basal rotation was more frequently observed in patients with ischemic response involving the anterior circulation territory. On the other hand, global apical rotation was not statistically different between normal patients and patients with IR-ExE. Previous studies have reported a lack of worsening of apical rotation during stress-induced ischemia or short duration of ischemia.15,16 The explanation is that subendocardial ischemia leads to an unopposed function of subepicardial fibers.16 Subendocardial fibers produce clockwise myocardial motion as opposed to counterclockwise motion caused by the subepicardial fibers. Because exercise may mainly lead to subendocardial ischemia in patients with CAD, a preponderance of subepicardial function might increase counterclockwise apical motion, as in our study. In this regard, apical rotation and LV-Tor increased after brief periods of ischemia (10 s) in animals, with further deterioration after more prolonged ischemia (30 s).16 In addition, older patients who may have fibrosis limited to the endocardium, have increased apical rotation.17 In contrast, impairment of apical rotation and LV-Tor has been observed after transmural ischemia in animals16 or during angioplasty.18 In a study in pigs, apical rotation decreased after ∼60% reduction of LAD perfusion pressure.19 On the other hand, LV-Tor increases with stimulation with dobutamine in the absence of ischemia.7 Therefore, increased apical rotation is expected when the disease (due to ischemia, necrosis or fibrosis) is limited to the subendocardium. Further ischemic burden with transmural ischemia or transmural necrosis would result in decreased apical rotation.
Explanations for reduced basal rotation at exercise in patients with ischemia are more intriguing. In a study by Bansal et al,15 patients with ischemia either in the anterior or inferior territories had impaired basal rotation during stress with dobutamine. We observed similar results in our patients with IR-ExE involving the anterior circulation territory. The study by Bansal et al also raised the question of why apical rotation was not reduced in patients with anteroapical ischemia and why basal rotation was limited (leading to a blunted clockwise motion). As a plausible explanation, the authors suggested that it might be due to differences in the ease with which the base and the apex rotate, the base being more restricted when there is worsening of twisting.2 Taking together the published studies in patients with myocardial infarction that measured basal rotation, it appears that basal rotation is impaired in inferior or inferolateral infarcts.5,6,15 In patients with anterior infarction, the results have been conflicting, with at least 3 studies founding impaired clockwise basal rotation,5,6,15 and another reporting basal rotation to be larger in anterior infarction than in inferior infarction and normal individuals.4 Therefore, basal rotation responses could differ, depending on the existence of necrosis or ischemia and their location.
Left Ventricular-torsion and Stress in Previous StudiesOnly a few studies have addressed the role of exercise on LV-Tor.20–22 In healthy young men, exercise on a bicycle increased LV twist from 8.8° (3.8°) to 12.8° (6.6°),20 which is higher than the values we obtained in our patients with normal ExE (an increase from 6.0° [2.5°] to 7.9° [3.9°]), which could probably be explained by differences in the methods of stress, the patients’ characteristics, and the technology employed. On the other hand, LV twist decreased after strenuous exercise provoked by a marathon run from 8.3° (5.1°) to 6.4° (3.9°).21
In patients with heart failure and normal LVEF, apical rotation was diminished at rest and at postexercise compared with that in the control group, although the increase in apical rotation with exercise did not differ from that of the control group,14 which agrees with our results. The increase of LV-Tor during exercise was also blunted in heart transplant recipients.23
In the only study similar to ours in which the effect of ischemia on LV-Tor was investigated by dobutamine stress echocardiography,15 ischemia had no marked effect on LV-Tor, although, as in our study, patients with anteroapical ischemia had blunted basal rotation at exercise. The mean global Torsion in this study was 3.05° at peak doses of dobutamine in patients with anteroapical ischemia and was 4.65° in patients without ischemia, which was not statistically different, probably due to the small number of patients included.
LV-Tor has been considered as a measurement of LV global function, and therefore it should be expected to decrease in the IR-ExE group, as LVEF decreased during exercise in this group (from 65° [8°] to 61° [12°]), in contrast with the values in the normal group (from 67° [8°] to 70° [10°]). However, LV-Tor at exercise was similar in both groups, suggesting a compensatory role of LV-Tor in patients with IR-ExE who had lower LVEF at exercise. In this regard, a study comparing normal individuals and football players demonstrated lower LVEF and higher LV-Tor in the former.24
Clinical ImplicationsAccording to our results, a normal increase in apical rotation during exercise may be observed even in patients with an extensive IR-ExE. Thus, vigorous apical counterclockwise rotation does not seem to rule out ischemia. On the other hand, a switch from normal clockwise basal rotation to a counterclockwise rotation might be a marker of ischemia. However, the absolute change in values in our study group—and hence the intergroup differences—were small.
LimitationsThe main limitation of this study is that the diagnosis of CAD was based on ExE, instead of coronary angiography, which was performed selectively in a subset of patients with abnormal results and in those with normal results and progressive or persistent symptoms. Although good for assessing outcome, ExE is an imperfect tool for the diagnosis of CAD. This is illustrated by the finding that 5 of 14 patients in the nonischemic group who ultimately underwent angiography had angiographically significant CAD, whereas 10 of the 35 patients with positive results on ExE had non-significant CAD or normal coronary arteries. In addition, the angiogram analyses were performed using a qualitative approach and the invasive cardiology team was not blinded to the ExE results.
Although the transient nature of stress provoked by exercise likely caused subendocardial ischemia in our patients with IR-ExE, we cannot rule out transmural ischemia in some of them. As discussed, transmural ischemia may have a different effect on LV-Tor than subendocardial ischemia.15–19
Ideally, for intergroup comparisons of LV-Tor, patients should not be on beta-blockers, although only 11% were taking these medications at the time of the ExE. Other clinical factors may have influenced the results, such as age, hypertension, LV hypertrophy, and diastolic dysfunction. Although the latter 3 conditions were similar between groups, patients with IR-ExE were a mean of 8 years older than those without. Moreover, although the presence of wall motion abnormalities was an exclusion criterion, a total of 21 patients had a history of myocardial infarction, which could have also affected the results.
On the other hand, this is a selected group of patients with an acceptable echocardiographic window in which tracking was feasible. Limitations in the technique of speckle tracking, such as plane change due to respiration, have been reported and might have affected our measurements in some patients, particularly during exercise. The exact location of the basal and apical planes might differ from patient to patient. Small changes in the plane of assessment may lead to significantly different values 25 (ie, lower apical rotation at more basal planes). Furthermore, although LV-Tor assessed by speckle tracking has been well validated at rest with magnetic resonance, it has not been validated during exercise. Although there are concerns about the ability of speckle tracking to evaluate images at heart rates of 140∼150 bpm, these heart rates were similar to those obtained in previous stress studies with speckle imaging.15
CONCLUSIONSAssessment of LV-Tor at rest and at exercise is feasible only in selected patients. LV-Tor is similar in patients with and without an IR-ExE although the former exhibit impaired basal rotation, mainly those with involvement of the anterior circulation territory. Apical rotation was similar to that of patients with normal ExE. Explanations for and confirmation of these findings merit further research.
CONFLICTS OF INTERESTNone declared.
Display e-component
Video 1 Two-dimensional echocardiography in the 4- and 2-chamber views in the same patient as in Figure 2, at rest (top) and at peak exercise (bottom), showing a global ischemic response to exercise echocardiography. Heart rate was 57 bpm at rest and 111 bpm at peak exercise.
Video 2 Two-dimensional echocardiography in the basal and apical short-axis views in the same patient as in Figure 2, at rest (top) and at peak exercise (bottom). The impairment of basal rotation and improvement of apical rotation with exercise may even be detected visually.



![Basal rotation at rest and at peak exercise in patients with normal exercise echocardiography, and in patients with ischemic response to exercise echocardiography in the posterior, anterior or anterior+posterior coronary distribution territories. The median [interquartile range] values are shown. ANOVA, analysis of variance: NS, not significant. *Basal rotation at peak exercise in: normal vs anterior ischemia, P = .003; anterior + posterior vs. anterior ischemia, P = .05; posterior vs anterior ischemia, P = .06; other intergroups comparisons, P = NS. Basal rotation at rest and at peak exercise in patients with normal exercise echocardiography, and in patients with ischemic response to exercise echocardiography in the posterior, anterior or anterior+posterior coronary distribution territories. The median [interquartile range] values are shown. ANOVA, analysis of variance: NS, not significant. *Basal rotation at peak exercise in: normal vs anterior ischemia, P = .003; anterior + posterior vs. anterior ischemia, P = .05; posterior vs anterior ischemia, P = .06; other intergroups comparisons, P = NS.](https://static.elsevier.es/multimedia/18855857/0000006700000009/v1_201408291224/S1885585714000838/v1_201408291224/en/main.assets/thumbnail/gr3.jpeg?xkr=eyJpdiI6Ik11MUQ3WHR6cU8yeFIzbitRWGVnOUE9PSIsInZhbHVlIjoibjZOeURzV055bGwxcjhnZHRJa2I1T0lMZytSd2pMWWMxRld5L2MyT3Z1VT0iLCJtYWMiOiI1OWEzZmVlZDEwZjA2OTMwYmJkM2RjZmQ2ODY1YTI5OWMyNzkxNzZhOTdmYzA4ODI1NGVhYjUwZDViYzA2NTMwIiwidGFnIjoiIn0=)



