Heart transplant is the most effective treatment for advanced heart failure (aHF). Due to the shortage of donors, there is growing interest in bridge-to-transplant therapies, such as medication with inotropic drugs.
Levosimendan is an inodilator drug whose active metabolite, OR-1896, has a prolonged action extending beyond the time of administration. Cycles of intermittent levosimendan (CIL) infusion have been shown to have clinical and hemodynamic benefits and to improve neurohormonal markers.1,2 However, CIL therapy has been linked to a worrying risk of ventricular arrhythmia during infusion.2 The main goal of the current study was to analyze the safety of outpatient CIL as a bridge to transplant.
We performed a prospective observational analysis of aHF patients3 included in a CIL program while on the heart transplant waiting list (HTWL) between January 2016 and May 2018. The initial 24-hour cycle was administered with electrocardiographic monitoring during a hospital admission. Infusion was begun at 0.1μg/kg/min, and the infusion rate was increased to 0.2μg/kg/min after 1 hour if systolic blood pressure remained ≥ 80mmHg. Subsequent outpatient cycles were scheduled every 2 months with a standard 6-hour protocol including hourly blood pressure readings, preceded by an electrocardiogram and blood analysis. At the time of inclusion on the HTWL, patients underwent right heart catheterization (RHC), with subsequent hemodynamic evaluations every 6-12 months.4 All patients were carriers of an implantable cardioverter-defibrillator (ICD). Follow-up continued from the first infusion cycle until heart transplant, implantation of a left-ventricular assist device, death, or end of study. Major adverse events were symptomatic hypotension or systolic blood pressure <80mmHg, ventricular tachycardia during follow-up (defined as symptomatic or hemodynamically unstable sustained ventricular tachycardia [>30 s]), and death.
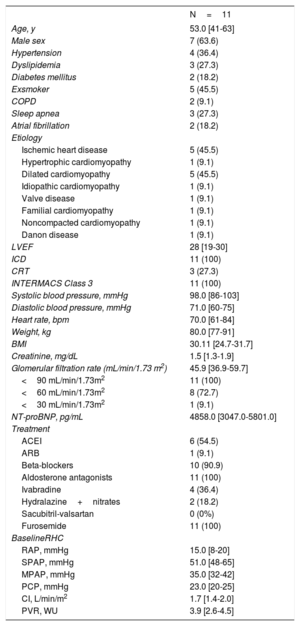
A total of 11 patients were included on the HTWL; all were in INTERMACS Class 3, 63.6% (7) were men, and the median age was 53 years [interquartile range, 41-63 years] (table 1). The median follow-up was 6 months [4-12 months], and the median number of infusion cycles during follow-up was 12 [8-25]. Only 1 patient had symptomatic hypotension during the treatment (systolic blood pressure, 70mmHg), which was resolved by reducing the infusion rate to 0.1μg/kg/min. None of the patients had ventricular arrhythmias during drug infusion, and there were no episodes of ventricular tachycardia during ICD interrogation. None of the patients died during the study period.
Baseline characteristics
| N=11 | |
|---|---|
| Age, y | 53.0 [41-63] |
| Male sex | 7 (63.6) |
| Hypertension | 4 (36.4) |
| Dyslipidemia | 3 (27.3) |
| Diabetes mellitus | 2 (18.2) |
| Exsmoker | 5 (45.5) |
| COPD | 2 (9.1) |
| Sleep apnea | 3 (27.3) |
| Atrial fibrillation | 2 (18.2) |
| Etiology | |
| Ischemic heart disease | 5 (45.5) |
| Hypertrophic cardiomyopathy | 1 (9.1) |
| Dilated cardiomyopathy | 5 (45.5) |
| Idiopathic cardiomyopathy | 1 (9.1) |
| Valve disease | 1 (9.1) |
| Familial cardiomyopathy | 1 (9.1) |
| Noncompacted cardiomyopathy | 1 (9.1) |
| Danon disease | 1 (9.1) |
| LVEF | 28 [19-30] |
| ICD | 11 (100) |
| CRT | 3 (27.3) |
| INTERMACS Class 3 | 11 (100) |
| Systolic blood pressure, mmHg | 98.0 [86-103] |
| Diastolic blood pressure, mmHg | 71.0 [60-75] |
| Heart rate, bpm | 70.0 [61-84] |
| Weight, kg | 80.0 [77-91] |
| BMI | 30.11 [24.7-31.7] |
| Creatinine, mg/dL | 1.5 [1.3-1.9] |
| Glomerular filtration rate (mL/min/1.73 m2) | 45.9 [36.9-59.7] |
| <90 mL/min/1.73m2 | 11 (100) |
| <60 mL/min/1.73m2 | 8 (72.7) |
| <30 mL/min/1.73m2 | 1 (9.1) |
| NT-proBNP, pg/mL | 4858.0 [3047.0-5801.0] |
| Treatment | |
| ACEI | 6 (54.5) |
| ARB | 1 (9.1) |
| Beta-blockers | 10 (90.9) |
| Aldosterone antagonists | 11 (100) |
| Ivabradine | 4 (36.4) |
| Hydralazine+nitrates | 2 (18.2) |
| Sacubitril-valsartan | 0 (0%) |
| Furosemide | 11 (100) |
| BaselineRHC | |
| RAP, mmHg | 15.0 [8-20] |
| SPAP, mmHg | 51.0 [48-65] |
| MPAP, mmHg | 35.0 [32-42] |
| PCP, mmHg | 23.0 [20-25] |
| CI, L/min/m2 | 1.7 [1.4-2.0] |
| PVR, WU | 3.9 [2.6-4.5] |
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CI, cardiac index; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter-defibrillator; LVEF, left-ventricular ejection fraction; MPAP, mean pulmonary arterial pressure; NT-proBNP, N-terminal pro-brain natriuretic peptide; PCP, pulmonary capillary pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RHC, right heart catheterization; SPAP, systolic pulmonary arterial pressure.
Data are expressed as No (%)or median [interquartile range].
During the CIL infusion program, 6 patients (54.5%) had at least 1 admission for decompensated heart failure, and 2 patients (18.2%) were admitted twice during this period. These figures are significantly lower than for the same length of time before initiation of levosimendan therapy, when 10 patients (90.9%) had at least 1 admission and the maximum number of single-patient admissions was 6. The median number of admissions in the CIL and pre-CIL periods were 1.0 [0-1] vs 2.0 [1-4] (P=.02).
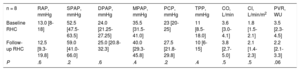
Of the cohort, 8 patients (72.7%) underwent RHC during follow-up, a median 8 months [7.1-9.9 months] after the baseline RHC. Parameters were stable between baseline and follow-up RHC, with only pulmonary vascular resistance showing a downward trend (table 2).
Comparison of hemodynamic parameters at baseline and during follow-up
| n = 8 | RAP, mmHg | SPAP, mmHg | DPAP, mmHg | MPAP, mmHg | PCP, mmHg | TPP, mmHg | CO, L/min | CI, L/min/m2 | PVR, WU |
|---|---|---|---|---|---|---|---|---|---|
| Baseline RHC | 13.0 [8-18] | 52.5 [47.5-63.5] | 24.0 [21.25-27.25] | 35.5 [31.5-41.0] | 23 [20-25] | 11 [8.5-18.0] | 3.6 [3.0-4.1] | 1.8 [1.5-2.1] | 3.5 [2.3-4.5] |
| Follow-up RHC | 12.5 [9.3-19.8] | 59.0 [41.0-66.0] | 25.0 [20.8-32.3] | 40.0 [29.3-45.8] | 27.5 [21.8-29.8] | 10 [6-15] | 3.8 [2.7-5.0] | 2.1 [1.4-2.3] | 2.2 [2.1-3.3] |
| P | .6 | .2 | .6 | .4 | .2 | .4 | .5 | .5 | .06 |
CI, cardiac index; CO, cardiac output; DPAP, diastolic pulmonary arterial pressure; MPAP, mean pulmonary arterial pressure; PCP, pulmonary capillary pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RHC, right heart catheterization; SPAP, systolic pulmonary arterial pressure; TPP, transpulmonary pressure.
Data are expressed as median [interquartile range].
At the time of the last levosimendan infusion, 7 patients (63.6%) were in INTERMACS Class 3 and 4 (36.4%) were in INTERMACS Class 2. All patients had subjective clinical improvement, and treatment was suspended in 1 patient. Of the patients, 9 (81.8%) underwent heart transplant, 2 of them (22.2%) in an emergency situation.
There were no statistically significant differences between baseline and end of follow-up concentrations of N-terminal pro-brain natriuretic peptide (NT-proBNP) (4858.0 [3047-5801] pg/mL vs 3407.0 [2188-4853] pg/mL; P=.3). Similarly, there were no differences between baseline and end of follow-up glomerular filtration rate (45.9 [36.9-59.7] mL/min/1-73m2 vs 47.0 [43.6-105.0] mL/min/1-73m2; P=.3).
This study examined a cohort of patients with aHF who received CIL as a bridge to heart transplant. Follow-up was longer than in previous reports,1,2 and ICDs were interrogated periodically, allowing analysis of levosimendan safety in patients included on a HTWL. Only 22% of the patients required an emergency heart transplant, contrasting with emergency transplant rates of 64% and 44% for HTWL patients in European and Spanish registries, respectively, in 2017.5,6 These data indicate that CIL is a practical bridge-to-transplant option.
Levosimendan infusion was safe in all patients, with no incidents of ventricular arrhythmia recorded during treatment or follow-up; however, the sample size is too small to allow definitive conclusions. Nevertheless, our results are important, since the prolonged action of the drug means that beneficial and adverse effects will not be limited to the infusion, but will also manifest in the days afterwards. The most concerning adverse effects are ventricular arrhythmias, but to our knowledge, no previous study has analyzed the occurrence of arrhythmias in the postinfusion period. Moreover, the heart failure admission rate in our cohort was lower than that reported in previous studies.1,2
FUNDINGThis study was supported by the Instituto de Salud Carlos III and the European Regional Development Fund (ERDF) through the Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV).
CONFLICTS OF INTERESTJuan F. Delgado has delivered presentations at Orion Pharma conferences and has participated in clinical trials funded by Orion Pharma. Javier de Juan and Inés Ponz have delivered presentations at Orion Pharma conferences.