Keywords
INTRODUCTION
A number of studies have shown the benefits of percutaneous coronary intervention (PCI) with systematic stenting in the setting of ST-elevation acute myocardial infarction (STEMI).1-3 However, the promising initial outcomes are limited in the medium and long term by restenosis.4 Thus, drug eluting stents (DESs) have been incorporated into the treatment of this group of patients, although there is little medium- and long-term experience. Furthermore, experience with DESs in patients with STEMI is currently still limited as such patients tend to be excluded from clinical trials.
The objective of this study was to verify the long-term safety and efficacy of DESs in a population of patients with STEMI compared to a similar group treated with bare-metal stents (BMSs) in normal clinical practice.
METHODS
This was a single-center cross-sectional observational study which, at 2 predefined times, compared 2 patient groups: one group comprised prospectively included patients with STEMI who underwent PCI with placement of 1 or more DESs, while the other group comprised retrospectively included patients of similar characteristics implanted with BMSs in the immediately preceding period.
Outcome Measures
The primary outcome measures were: a) incidence at 12 months of the need for target lesion revascularization (TLR); and b) rate of angiographically documented restenosis (1 year after the intervention). The secondary objectives were: a) the incidence of a composite outcome of major adverse cardiac events (MACE) defined as death, nonfatal reinfarction, or need for new revascularization at 12 months; b) incidence of acute thrombosis (in the first 24 hours), subacute thrombosis (between 1 and 30 days), and late thrombosis (after 30 days); and c) late lumen loss.
Definitions
Cardiac death was defined as cardiac (AMI, CHD, fatal arrhythmias), death with no cause reported, death of unknown cause, and procedure related death. Target lesion revascularization was defined as a new intervention, whether surgical or percutaneous, of the lesion that had previously been successfully treated. Reinfarction was defined as the appearance of new diagnostic Q-waves in at least 2 adjacent leads in the ECG or a clinical picture compatible with creatine kinase (CK) elevation and a CK muscle-brain fraction (CK-MB) twice as high as the normal reference range of the laboratory. Stent thrombosis was defined as an acute coronary syndrome with complete occlusion or a thrombus limiting flow in the successfully treated target vessel, documented by angiography, or, in absence of angiographic confirmation, the presence of AMI in the territory irrigated by the treated vessel. Restenosis was taken to be the presence during angiographic follow-up of >50% stenosis in the stented segment and the 5 mm of vessel proximal and distal to the stent.
Inclusion and Exclusion Criteria
We included patients with STEMI treated with primary PCI or rescue PCI in whom 1 or more stents were implanted. Exclusion criteria were as follows: patients in cardiogenic shock, reference diameter of the AMI culprit artery <2.5 mm or >4 mm, lesions of the left main coronary artery, and those in which the clinical and angiographic follow-up protocol could not be applied.
Procedure and Antithrombotic Treatment
The PCI was done according to the generally accepted guidelines and in accordance with the judgment of each operator. The use of antithrombotic and antiplatelet therapy during the procedure was done according to the normal laboratory procedures. After the procedure, the patients followed a regimen of 100 mg/d of aspirin indefinitely and 75 mg/d of clopidogrel for 1 month in the case of those implanted with BMSs and at least 12 months in the case of those implanted with DESs.
Follow-up
The patients were scheduled for visits at 30 days, 6 months, and 1 year after the procedure. The angiographic study was done sometime after 1 year had elapsed (12-15 months after the procedure). Only in cases of recurrence of symptoms was the angiographic study done sooner. However, in these cases, if restenosis was not documented angiographically, the control angiographic study was still performed after 1 year.
Statistical Analysis
A descriptive analysis of both groups was undertaken, presenting the frequency distribution for the qualitative variables and the mean (SD) for the quantitative ones. The Kolmogorov-Smirnov test was done to determine whether the distribution was normal. To compare the main outcome variables, the c2 test was used for categoric variables and the ANOVA and Student t test for continuous ones, for paired, or independent samples as appropriate (according to whether the distribution was normal; if not, the nonparametric Wilcoxon or Mann-Whitney U tests were used, respectively). The associations were considered statistically significant for P values of less than .05. For all variables, 95% confidence intervals were calculated. A multivariate analysis was undertaken using a Cox proportional risk regression model to identify predictors of adverse events during follow-up. In the analysis, all variables with group differences with an associated value of < P value of less than .1 in the univariate analysis were included. The Kaplan-Meier method was used to analyze the event free time during follow-up and the log rank test was used to compare the curves of the 2 groups. The entire statistical analysis was carried out using the SPSS software package, version 12.0.
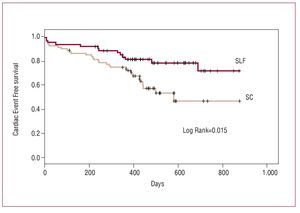
Figure 1. Kaplan-Meier cardiac event-free survival curves (acute myocardial infarction and/or need for repeat revascularization) in both groups at the end of the follow-up period. BMS indicates group treated with bare-metal stents; DES, group treated with drug-eluting stents.
RESULTS
Between September 2003 and July 2005, a total of 134 patients with STEMI underwent PCI in our center. In 92 cases, 1 or more DESs were implanted during the procedure, and 81 of these met the inclusion criteria (DES group). The other group—obtained retrospectively— comprised 82 patients with similar characteristics who had undergone PCI and been implanted with a BMS (BMS group) in the immediately preceding period, between July 2001 and August 2003. The baseline characteristics of the patients are shown in Table 1 according to the type of stent used. There were no significant differences in the baseline clinical characteristics assessed, except for the mean (SD) age, which was lower in the DES group (56 [11] years vs 60 [12] years; P=.02). The most frequent indication for intervention in both groups was primary PCI.
Table 2 shows the angiographic characteristics of the culprit lesion and the characteristics of the procedure. In addition to the AMI culprit lesion, a further 5 lesions in the BMS group and a further 16 lesions in the DES group (10 with DES and 6 with BMS) were treated. In total, 97 lesions were treated in the DES group and 87 in the BMS group, with a similar mean number of lesions treated in both groups (1.19 vs 1.06 lesions/patient; P=.11). The percentage of complex lesions (types B2 and C) was greater in the DES group (67.9%) than in the BMS group (54.8%). In addition, in the DES group, 19 bifurcation lesions (23.4%) were treated compared to just 3 (3.6%) in the BMS group. There were no differences between groups in terms of baseline TIMI flow grade. The angiographic quantification of the AMI culprit lesion did not show significant differences between groups in terms of length of lesion and reference vessel diameter; however, in the DES group a higher percentage of vessels with diameter less than 2.8 mm was found (33.3% vs 10.9%; P=.001).
After the procedure, TIMI flow grade 2-3 was achieved in the AMI culprit artery in more than 96% of the patients in both groups. In total, 103 stents were implanted in the DES group (73 were sirolimus-eluting stents and 30 were paclitaxel-eluting stents) and 90 in the BMS group, with a proportion of stents/lesion of 1.3 and 1.1, respectively (P=.008). The total length covered by the stent was also significantly greater for the DES group. In the DES group, direct stenting without predilation was done more often (37% vs 1.2%; P<.001). Also, the use of glycoprotein IIb/IIIa inhibitors was more often related to placement of DESs (61.3% vs 29.3%; P<.001).
Clinical Follow-up
Follow-up was completed by all patients. Table 3 shows the in-hospital adverse events and adverse events at 6 months and 1 year. Mortality at 1 year was 7.3% in the BMS group and 2.5% in the DES group (P=.15). Although there were no significant differences in TLR at 6 months, at 1 year, this outcome occurred significantly less often in the DES group (8.6% vs 23.2%; HR = 0.31; 95% confidence interval [CI], 0.12-0.79; P=.001), that is, an absolute decrease of 14.6% and a relative one of 63% was found. There were also fewer MACEs at 1 year in the DES group (13.6% vs 31.7%; HR = 0.33; 95% CI, 0.15-0.74; P=.006).
Five cases of stent thrombosis were reported, 3 in the DES group (2 with sirolimus-eluting stents and 1 with a paclitaxel-eluting stent) and 2 in the BMS group, corresponding to incidences of 3.7% and 2.4%, respectively (P=.64). The 2 cases of DES thrombosis occurred on day 1 and at 7 days after the intervention. In this latter case, the patient had voluntarily stopped taking antiplatelet medication after discharge.
Although follow-up was initially planned for 1 year, the final follow-up was longer (543 [187] days in the DES group and 434 [156] days in the BMS group). After 1 year, and until the end of follow-up, 2 patients died in the BMS group and none in the DES group. Thus, at the end of follow-up, mortality in patients treated with DESs was 2.5% compared to 9.8% in the BMS group (P=.053), and TLR was done in 9.9% in the DES group and 29% in the BMS group (P=.002). In this period, a patient who had received a sirolimus-eluting stent suffered late stent thrombosis 2 weeks after stopping clopidogrel and 14 months after the procedure. At the end of the follow-up period, the MACE-free survival in the DES group was 84.1% compared to 61% in the BMS group (log rank test, P=.0007) (Figure).
In the multivariate analysis using the Cox regression model, which included all confounding variables between groups and those that had a P value <.01 in the univariate analysis (Table 4), the use of DES was an independent predictor of TLR (HR = 0.10; 95% CI, 0.03-0.32; P<.001) and MACEs at 1 year (HR = 0.18; 95% CI, 0.06-0.57; P=.004).
Angiographic Follow-up
Angiographic follow-up was done in 83% of the patients in the DES group at a mean of 387 (21) days after the procedure and in 79% of the BMS group at 369 (48) days after the initial procedure. Table 2 shows the values obtained in the angiographic quantification. The primary angiographic outcome measure—restenosis rate—was 13.8% in the DES group compared to 30.9% in the control group, corresponding to an absolute reduction in restenosis of 17% and a relative reduction of 55% (HR = 0.36; 95% CI, 0.15-0.87; P=.02). Late lumen loss was also less in the DES group than the BMS group (0.43 vs 0.9 mm; P=.01).
DISCUSSION
The results of this study demonstrate the safety of DES placement in patients with STEMI, with significantly lower restenosis rates and less need for repeat revascularization procedures compared to BMS.
Patient and Procedure Characteristics
The groups in our study comprised patients for whom primary or rescue PCI was indicated in accordance with the clinical guidelines provided that catheterization laboratories were available at the time. The clinical and angiographic baseline characteristics are those expected for patients with STEMI. Of note is the higher prevalence of diabetic patients than in other studies of STEMI such as the RESEARCH and PASSION studies, in which only 12.5% and 11%, respectively, of the patients treated with DES were diabetic.5,6 Moreover, and probably as a result of this higher percentage of diabetic patients, the reference vessel diameter in our DES group was less than that of patients treated with paclitaxel-eluting stents in the PASSION study (2.95 and 3.13 mm).6 We should also bear in mind that the relationship between vessel size and restenosis and stent thrombosis as vessel size decreases has been well established in the literature.7 Some aspects of the procedure differ between the 2 groups of our study, in particular because the patients were treated at different times. Thus, administration of glycoprotein IIb/IIIa inhibitors was greater in the DES group in accordance with the current clinical guidelines on antithrombotic therapy.8,9 The percentage use of such therapy in our study is lower than in other studies,6,10 probably because patients undergoing rescue PCI were included in our population. On the other hand, direct stenting was much more frequent in the DES group than in the BMS group because, during this period, this was the recommended technique for angiographically documented thrombotic lesions with no calcification and in which thrombus aspiration devices were not used, as this is associated with a lower incidence of low or no reflow and a better resolution of the ST segment.11-13 Similarly, the length of the segment covered by the stent was also greater in the group of patients with DESs (22 vs 18 mm; P=.001). Currently, when DESs are used, it is recommended that either end of the stent corresponds to healthy segments, that is, longer stents should be used.14,15
Clinical Outcomes
During the first 6 months of follow-up, there were no significant differences in mortality, MACEs, or TLR between patients who had STEMI treated with DESs and those treated with BMSs. However, after 1 year, the use of DESs was associated with a decrease in TLR (8.6% vs 23.2%; P=.01) and MACEs (13.6% vs 31.7%; P=.006). The benefit observed in MACEs at 1 year was due to the decrease in the need for repeat revascularization, as no differences in mortality or incidence of nonfatal reinfarction were found.
The rate of TLR at 6 months in our patients with DESs is high compared to the RESEARCH registry, in which it was only 1%.5 However, the STRATEGY study reported a similar TLR rate to us at 8 months, with a clear benefit in favor of the sirolimus+tirofiban-eluting stent compared to a BMS+abciximab (6% and 20%). In that study, the decrease in TLR also translated into a significant reduction in MACEs (18% vs 32%; P=.04).16 In the aforementioned TYPHOON study, the TLR at 12 months was 6% in the sirolimus-eluting stent group and 13% in the BMS group (P<.001).10 Similar findings have been reported recently for the SESAMI (a study done in 320 patients in a primary PCI setting, comparing sirolimus-eluting stents with BMSs). In that study, the MACE rate at 12 months was significantly lower in the sirolimus-eluting stent group (7% vs 17%; P<.05).17 Probably, the tendency for most of the events to occur from the sixth month onwards meant that the analysis of the later outcomes revealed higher rates of event. However, in the PASSION study, there were no significant differences at one year for the composite endpoint (although a trend towards a lower incidence in the DES group could be discerned) while the TLR was similar (5% in the paclitaxel-eluting stent group and 8% in the BMS group).6 We should highlight that the incidence of events in the BMS group of this study was low, probably because a low percentage of diabetic patients (12%) were enrolled and patients had larger target vessel diameters — 3.21 mm versus 2.84 mm in the TYPHOON and 2.95 mm in our study. Moreover, that study did not carry out an angiographic follow-up, and doing systematic angiographic studies, as in our case, might increase the number of indications for intervention due to angiographic restenosis in absence of symptoms or evidence of ischemia.
In our study, we found that the beneficial effects of DESs are maintained during the long-term follow-up. Although conducted in a different setting, similar results have been reported in the metaanalysis of Kastrati et al,18 who included 14 trials that compared sirolimus-eluting stents with BMSs, with a follow-up period of 5 years. They found that the use of sirolimus-eluting stents was associated with a sustained decrease in the need for repeat intervention, and mortality and nonfatal AMI were similar to those observed in BMSs.
Angiographic Follow-up
Approximately 80% of the patients in both groups underwent angiographic studies. The findings showed clear advantages in favor of DES in all the angiographic variables analyzed. However, our restenosis rate with DESs is greater than those observed in the few studies published in this setting that undertook an angiographic follow-up. Surprisingly, in some of these, such as the RESEARCH registry, the authors did not report any cases of restenosis at 6 months follow-up in the subgroup of 96 patients with STEMI who were treated with sirolimuseluting stents.5 In the STRATEGY and TYPHOON studies with angiographic follow-up at a somewhat later point— 8 months—restenosis was significantly greater in patients treated with BMSs than those treated with sirolimus-eluting stents (36% vs 9% and 20% vs 4%, respectively).10,16 The SESAMI study reported a lower restenosis rate in the DES group at 12 months (9% vs 21%; P<.05).17 The restenosis rates with DES in these 4 studies (0%-9%) are lower than the ones we found and are probably related to a different patient selection, less rigorous evaluation of the vessel diameter in the AMI setting (generally underestimated), and the later angiographic control in our study (more than 1 year after the intervention compared to 6-12 months in the aforementioned studies).
Stent Thrombosis
The incidence of stent thrombosis in patients with STEMI seems to be higher than in other settings.19 In our study, the incidence of acute and subacute thrombosis was 3.7% in the DES group and 2.4% in the BMS group (P=.32). Moreover, in this period, a patient who had received a sirolimus-eluting stent suffered late stent thrombosis at 14 months, probably due to discontinuation of clopidogrel.
However, although the incidence of thrombosis is higher, DESs implanted in patients with STEMI do not seem to be associated with a higher rate of thrombosis than when BMSs are used.6,10,20 It is likely that the hemodynamic situation of this type of patient, with increased prothrombotic activity, is the reason for this increased incidence. For this reason, in these patients with STEMI, we should be more demanding when optimizing our implantation technique and extremely careful with the antithrombotic and antiplatelet regimens to minimize the possibility of stent thrombosis.
Limitations of the Study
The main limitation of this study was that patients were not randomly assigned. This explains some of the differences in baseline characteristics between the study group and the control group taken retrospectively from an earlier period. However, the populations analyzed may be representative of the "real world" of interventional cardiology. Furthermore, as this was a nonrandomized study, the prospective inclusion of patients in the DES group was done such that a selection of patients at higher risk of stenosis occurred in this group, thereby introducing a bias in the clinical and angiographic findings. In addition, the small sample size may be responsible for the failure to obtain statistically significant results despite the differences observed.
Finally, the results of this study were obtained from a single center, with a small volume of interventional procedures, and so they are only applicable to similar centers.
CONCLUSIONS
DES placement in patients with STEMI who undergo PCI—while not appearing to influence mortality or the possibility of reinfarction compared to BMS placement— does provide clear angiographic benefit. Thus, the rate of restenosis is reduced, which translates into a significant decrease in MACEs after 1 year of follow-up as a result of the decrease in the need for new revascularization of the target lesion. Although the risk of stent thrombosis in these patients with STEMI seems to be high, there are no differences between the 2 types of stent.
ABBREVIATIONS
BMS: bare-metal stent
DES: drug-eluting stent
MACE: major adverse cardiac events
PCI: percutaneous coronary intervention
STEMI: ST-elevation acute myocardial infarction
TLR: target lesion revascularization
SEE EDITORIAL ON PAGES 346-8
Correspondence:
Dra. A.M. Planas del Viejo.
Servicio de Cardiología. Hospital General Universitario. Avda. Tres Cruces, 2. 46014 Valencia. España.
E-mail: planas_ana@gva.es
Received June 17, 2007.
Accepted for publication January 8, 2008.