In patients who have undergone repair of coarctation of the aorta, hemoptysis is a worrying symptom that should lead to suspicion of an underlying thoracic aortic aneurysm complicated with an aortobronchial fistula.1 Given the ominous prognosis associated with this complication, we wanted to study its causes and outcomes in this context.
We present the first case series, to our knowledge, of patients with a history of coarctation of the aorta who developed hemoptysis years after repair, with an analysis of the causes, complications, treatment, and outcomes.
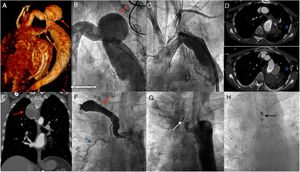
Of a cohort of 481 adult patients with coarctation of the aorta diagnosed in Hospital Universitario La Paz between 1999 and 2018, 357 underwent surgical repair (146 with patch aortoplasty). At long-term follow-up, 49 patients (10% of the series) developed aneurysms or pseudoaneurysms at the site of surgical aortoplasty of the descending aorta (descending aortic aneurysm was defined as dilatation greater than 150% of the diameter of the diaphragmatic aorta) and 3 patients developed aneurysms of the intercostal arteries. Of the entire cohort, 7 patients (1.5%) developed hemoptysis at follow-up; of these, 3 died. The characteristics and clinical outcomes of these patients are described in table 1. Of the 7 patients with hemoptysis, 2 had similar outcomes: 1 patient, after repeated, self-limiting episodes of hemoptysis due to an aortobronchial fistula, underwent successful surgery for the pseudoaneurysm (at that time endovascular treatment was not available) and died in the immediate postoperative period due to a further episode of massive hemoptysis following severe pulmonary hemorrhage; the other patient, with no previous history of hemoptysis, underwent an elective endovascular procedure to exclude the aneurysm with percutaneous implantation of 2 polytetrafluoroethylene stents. At 7 days postprocedure, after exclusion of the pseudoaneurysm at the aortoplasty site, the patient was readmitted with an episode of massive hemoptysis, in which severe bleeding was observed in the left lung adjacent to the pseudoaneurysm (figure 1A-D). An aortic endoprosthesis was placed, but the patient died due to multiorgan failure in the days following the pulmonary hemorrhage. Finally, the third patient, after an episode of massive hemoptysis secondary to an aortobronchial fistula, and despite successful emergency surgery to implant 2 vascular endoprostheses, died days later due to multiorgan failure. The other 4 patients in the series had repeated episodes of hemoptysis: 3 were treated with endovascular exclusion of the pseudoaneurysm, and the fourth, after percutaneous exclusion of an intercostal artery aneurysm, developed episodes of hemoptysis secondary to the presence of an aberrant bronchial artery, which was successfully embolized (figure 1E-H).
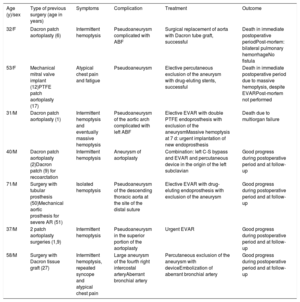
Characteristics of patients with hemoptysis, their treatment, and outcomes at follow-up
| Age (y)/sex | Type of previous surgery (age in years) | Symptoms | Complication | Treatment | Outcome |
|---|---|---|---|---|---|
| 32/F | Dacron patch aortoplasty (6) | Intermittent hemoptysis | Pseudoaneurysm complicated with ABF | Surgical replacement of aorta with Dacron tube graft, successful | Death in immediate postoperative periodPost-mortem: bilateral pulmonary hemorrhageNo fistula |
| 53/F | Mechanical mitral valve implant (12)PTFE patch aortoplasty (17) | Atypical chest pain and fatigue | Pseudoaneurysm | Elective percutaneous exclusion of the aneurysm with drug-eluting stents, successful | Death in immediate postoperative period due to massive hemoptysis, despite EVARPost-mortem not performed |
| 31/M | Dacron patch aortoplasty (1) | Intermittent hemoptysis and eventually massive hemoptysis | Pseudoaneurysm of the aortic arch complicated with left ABF | Elective EVAR with double PTFE endoprosthesis with exclusion of the aneurysmMassive hemoptysis at 7 d: urgent implantation of new endoprosthesis | Death due to multiorgan failure |
| 40/M | Dacron patch aortoplasty (2)Dacron patch (9) for recoarctation | Intermittent hemoptysis | Aneurysm of aortoplasty | Combination: left C-S bypass and EVAR and percutaneous device in the origin of the left subclavian | Good progress during postoperative period and at follow-up |
| 71/M | Surgery with tubular prosthesis (50)Mechanical aortic prosthesis for severe AR (51) | Isolated hemoptysis | Pseudoaneurysm of the descending thoracic aorta at the site of the distal suture | Elective EVAR with drug-eluting endoprosthesis with exclusion of the aneurysm | Good progress during postoperative period and at follow-up |
| 37/M | 2 patch aortoplasty surgeries (1,9) | Intermittent hemoptysis | Pseudoaneurysm in the superior portion of the aortoplasty | Urgent EVAR | Good progress during postoperative period and at follow-up |
| 58/M | Surgery with Dacron tissue graft (27) | Intermittent hemoptysis, repeated syncope and atypical chest pain | Large aneurysm of the fourth right intercostal arteryAberrant bronchial artery | Percutaneous exclusion of the aneurysm with deviceEmbolization of aberrant bronchial artery | Good progress during postoperative period and at follow-up |
ABF, aortobronchial fistula; AR, aortic regurgitation; C-S, carotid-subclavian; EVAR, endovascular repair of aneurysm; F, female; M, male; PTFE, polytetrafluoroethylene.
A-D: percutaneous treatment and exclusion of thoracic aortic pseudoaneurysm using a drug-eluting stent, with subsequent death of the patient due to hemoptysis. A: three-dimensional CT reconstruction of the pseudoaneurysm (red arrow). B: baseline angiography of the pseudoaneurysm (red arrow). C: angiography showing exclusion of the pseudoaneurysm with 2 polytetrafluoroethylene-coated stents. D: 2 CT images showing pulmonary hemorrhage (blue asterisks) adjacent to the stent, resulting in the death of the patient. E-H: percutaneous treatment and exclusion of intercostal artery aneurysm and embolization of aberrant bronchial artery. E: CT image showing the partially-thrombosed giant aneurysm of the intercostal artery (red arrow). F: baseline angiogram showing the intercostal artery aneurysm (red arrow) and the aberrant bronchial artery (blue arrow). G: percutaneous exclusion of aneurysm with device (white arrow). H: coil embolization (black arrow) of aberrant bronchial artery. CT, computed tomography.
Aortobronchial fistulas were first described in 1934 by Keefer and Malory.2 In 1962, Davey described the first successful surgical repair.3 These fistulas are caused by the thoracic aneurysm eroding the adjacent pulmonary tissue or bronchial tree. They are an uncommon condition, usually presenting with hemoptysis, which is generally recurrent and self-limiting, but which gradually increases in severity until massive hemoptysis occurs. Diagnosis is with imaging such as computed tomography, which shows an aneurysm or pseudoaneurysm, often with mural thrombus; only occasionally is extravasation of contrast to the lung seen.4 The treatment of choice is implantation of an aortic endoprosthesis.5,6 Currently, regular follow-up of these patients with imaging techniques allows the early diagnosis of aneurysms that can be repaired early and thus prevent the development of aortobronchial fistulas.
It should be noted that 2 patients in our series died due to hemoptysis in the week following successful repair of the pseudoaneurysm. Hypothetically, it is possible that after the exclusion of the aneurysm, the aortic and pulmonary anatomical arrangement may have changed and the adjacent lung tissue that was previously damaged started bleeding again, with the patient dying due to massive hemoptysis or multiorgan failure days later.
In conclusion, hemoptysis is a serious symptom in patients with repaired coarctation of the aorta that is usually due to the development of an aortobronchial fistula at the site of the repaired aneurysm or pseudoaneurysm. This complication requires early assessment and urgent endovascular treatment. Even with successful repair, close follow-up is recommended during the immediate postoperative period due to the risk of hemorrhage from the pulmonary tissue adjacent to the pseudoaneurysm, with potentially fatal outcome.
CONFLICTS OF INTERESTA. Sánchez-Recalde is associate editor of Revista Española de Cardiología.