In 1984, the percutaneous mitral valvuloplasty (PMV) technique of the Japanese surgeon Inoue was published, revolutionizing mitral stenosis treatment and, due to better results, supplanting the surgical technique.1 In the Euro Heart Survey of 2001, mitral stenosis was seen in 9.5% of 5001 patients.2 Of 112 patients who underwent a stenosis intervention, 34% received a percutaneous treatment.2 This intervention showed excellent results in subsequent studies with follow-up durations of up to 20 years.3 However, some valve restenosis patients later require valve replacement surgery; nonetheless, some patients could still be candidates for a repeat PMV. Because repeat PMV data are scarce in the literature3 and nonexistent in Spain, our objective was to determine the characteristics and clinical course of patients who underwent a repeat PMV in an extensive PMV series.
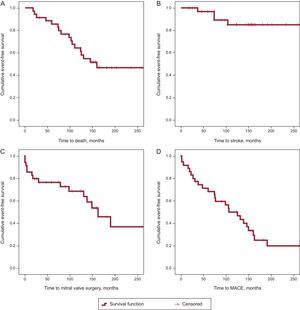
A total of 1138 consecutive PMV were retrospectively reviewed. These procedures were performed between 1988 and 2004 using the Inoue technique (with a single balloon containing 3 regions, initially in the form of an hourglass, the waist of the balloon is the least compliant part and opens the commissures). Clinical data were collected from the medical records and follow-up in the clinic or by telephone. Of the PMV, 35 repeat PMV were identified between 1989 and 2012. Of these, 5 were in men, and the mean patient age was 57.3 years (Table). Ten patients received a suboptimal PMV that required a second catheterization before 60 days. Overall, the median time to repeat PMV was 4.7 years (7.1 years if suboptimal PMV were excluded). The median follow-up was 10.8 years (Figure). One patient required urgent surgery after the repeat PMV due to procedure-related complications. During follow-up, 17 patients died (median survival, 8.1 years), 7 of cardiac causes (including 1 cardiac arrest and 2 related to cardiac surgery), 5 of noncardiac causes (2 due to bleeding, and 1 each due to stroke, kidney failure, and pancreatic cancer), and 5 of nonspecific causes.
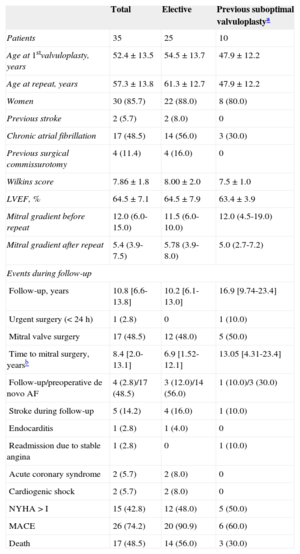
General Characteristics of Patients in the Entire Cohort and Divided According to the Intervention Type (Elective or Due to a Previous Failed/Suboptimal Valvuloplasty). The Events Registered During Follow-up Are Detailed According to the Above Groups
| Total | Elective | Previous suboptimal valvuloplastya | |
|---|---|---|---|
| Patients | 35 | 25 | 10 |
| Age at 1stvalvuloplasty, years | 52.4±13.5 | 54.5±13.7 | 47.9±12.2 |
| Age at repeat, years | 57.3±13.8 | 61.3±12.7 | 47.9±12.2 |
| Women | 30 (85.7) | 22 (88.0) | 8 (80.0) |
| Previous stroke | 2 (5.7) | 2 (8.0) | 0 |
| Chronic atrial fibrillation | 17 (48.5) | 14 (56.0) | 3 (30.0) |
| Previous surgical commissurotomy | 4 (11.4) | 4 (16.0) | 0 |
| Wilkins score | 7.86±1.8 | 8.00±2.0 | 7.5±1.0 |
| LVEF, % | 64.5±7.1 | 64.5±7.9 | 63.4±3.9 |
| Mitral gradient before repeat | 12.0 (6.0-15.0) | 11.5 (6.0-10.0) | 12.0 (4.5-19.0) |
| Mitral gradient after repeat | 5.4 (3.9-7.5) | 5.78 (3.9-8.0) | 5.0 (2.7-7.2) |
| Events during follow-up | |||
| Follow-up, years | 10.8 [6.6-13.8] | 10.2 [6.1-13.0] | 16.9 [9.74-23.4] |
| Urgent surgery (< 24 h) | 1 (2.8) | 0 | 1 (10.0) |
| Mitral valve surgery | 17 (48.5) | 12 (48.0) | 5 (50.0) |
| Time to mitral surgery, yearsb | 8.4 [2.0-13.1] | 6.9 [1.52-12.1] | 13.05 [4.31-23.4] |
| Follow-up/preoperative de novo AF | 4 (2.8)/17 (48.5) | 3 (12.0)/14 (56.0) | 1 (10.0)/3 (30.0) |
| Stroke during follow-up | 5 (14.2) | 4 (16.0) | 1 (10.0) |
| Endocarditis | 1 (2.8) | 1 (4.0) | 0 |
| Readmission due to stable angina | 1 (2.8) | 0 | 1 (10.0) |
| Acute coronary syndrome | 2 (5.7) | 2 (8.0) | 0 |
| Cardiogenic shock | 2 (5.7) | 2 (8.0) | 0 |
| NYHA > I | 15 (42.8) | 12 (48.0) | 5 (50.0) |
| MACE | 26 (74.2) | 20 (90.9) | 6 (60.0) |
| Death | 17 (48.5) | 14 (56.0) | 3 (30.0) |
AF, atrial fibrillation; LVEF, left ventricular ejection fraction; MACE, major adverse cardiac events (composite variable of death from any cause, mitral valve surgery, stroke, or mitral valve endocarditis); NYHA, New York Heart Association functional class.
The data are expressed as No. (%), mean±standard deviation, or median [interquartile range].
Long-term event-free survival curves (Kaplan-Meier). A: Probability of death. B: Stroke during follow-up. C: Need for mitral valve surgery. D: Composite variable of major adverse cardiac events (death, stroke, need for cardiac surgery, and endocarditis). MACE, major adverse cardiac events.
In patients who required surgery (48.5%), it was performed at a median of 54.14 months (range, 3.9-146.14 months) after the repeat PMV. In total, 5 patients required 2 surgical interventions; 14, only 1, and 16, none; of these, 10 were still alive at the end of follow-up.
When univariate analysis was performed, no significant differences were found in any variable (eg, sex, cardiovascular risk factors, previous commissurotomy, repeat PMV due to failed/suboptimal PMV), except proportionality in the age at the time of the repeat PMV regarding the need for surgery and occurrence of major cardiovascular adverse events.
The present work represents the longest repeat PMV series in Spain and one of the longest in the literature, with a follow-up of up to 25 years. Repeat PMV is an uncommon procedure, performed in 3% of patients who have previously undergone PMV. Nonetheless, notably, there was no clear difference in the long-term clinical course between elective repeat procedures and those repeat PMVs performed due to failure of the first intervention. The patients’ clinical course was reasonably good, especially considering that their mean age at the intervention was almost 60 years, and about half of the patients were free of major adverse cardiac events more than 8 years after the repeat PMV. Thus, some patients never require a cardiac surgical intervention and, of those who ultimately require the operation, repeat PMV manages to delay it by several years, which is in agreement with some published articles.3,4 These data indicate the safety and effectiveness of a classic, low-cost technique that has been widely used in our environment. Although PMV is not intended to compete with the treatment generally indicated after mitral restenosis, which is surgical replacement, there is little comparative evidence. Repeat PMV was suggested to be a reasonable long-term option in the single available report on PMV, a small series of patients reported by Aslanabadi et al4 (25 repeat PMVs and 22 valve replacements).
In addition, the use of mitral valvuloplasty is decreasing in many countries, such as the United States and even Spain, where, despite being the most frequent percutaneous valvuloplasty technique, the number of procedures has decreased from 254 in 2012 to 240 in 2013.5
The 2012 European Guidelines recommend PMV for symptomatic patients with severe stenosis and favorable anatomy in the absence of atrial thrombus or moderate-to-severe mitral regurgitation. The same document recommends repeat PMV for selected patients, particularly those whose mitral restenosis mechanism is secondary to commissural fusion, with favorable anatomy, and who are not surgical candidates (palliative care).6
The present study is inherently limited by its retrospective design and the inclusion of patients who underwent PMV many years ago, some more than 20 years ago. Repeat PMV is a little used intervention that allows mitral valve replacement surgery to be avoided or delayed by several years in selected patients who have already undergone a percutaneous commissurotomy.
.