Drug-eluting stents (DES) met with considerable initial success.1 However, it was not long before concerns arose about the inflammation and delayed healing caused by drug-releasing polymers2 and the risk of late stent thrombosis.3,4 Various strategies have been developed to improve the safety of DES without compromising their efficacy in preventing restenosis. These included more biocompatible permanent polymers,5 biodegradable polymers,6 and polymer-free technology.7 However, the reputation of bare-metal stents as safer devices has not disappeared and efforts have focused on the development of special passive8 or bioactive coatings9 that may improve both the safety and efficacy of bare-metal stents. In this context, several iterations of strut material (from stainless steel to cobalt chromium), strut thickness (down to 81μm), stent architecture (currently a helicoidal design) and coating technology (coating with titanium nitride oxide by plasma enhanced vapor deposition of titanium in a gas mixture of nitrogen and oxygen) led to the development of the Titan-2 (Hexacath, France)10 and, ultimately, the OPTIMAX (Hexacath, France) stent.11
There is no doubt that randomized, sufficiently powered clinical trials with long-term follow-up are the best way to assess the relative advantages of stent technologies in patients with coronary artery disease. However, considerable resources and a long time interval are required before the true advantages can be known. Therefore, surrogate parameters that can be evaluated in a feasible sample size of patients over a shorter period may be of considerable help if they are able to predict late thrombotic events. In this regard, intravascular optical coherence tomography (OCT) with its excellent spatial resolution is a valuable investigation technology. Uncovered or malapposed stent struts as well as neoatherosclerosis were found to underlie stent thrombosis to a degree that was dependent on the time point of its occurrence (subacute, late, and very late).12
In a recent article published in Revista Española de Cardiología, Sia et al.13 report the results of the OPTIMAX-OCT study, a randomized study that compared OPTIMAX, a bioactive stent (BAS), with SYNERGY (Boston Scientific Corporation, United States), a biodegradable polymer-based everolimus-eluting stent (EES) in patients presenting with acute coronary syndrome (ACS) and de novo lesions in a native coronary artery. The primary endpoint of the study was the percentage of uncovered struts per patient, and the coprimary endpoint was the percentage of malapposed struts per patient, which were assessed with the use of a single OCT study in 2 separate cohorts corresponding to 2 time points: cohort A (52 patients) at 30 days and cohort B (30 patients) at 6 months. Apparently, it is a stand-alone study, independent of the TIDES-ACS (Comparison of Titanium-Nitride-Oxide Coated Bio-active-Stent [Optimax] to the Drug [Everolimus]-Eluting Stent [Synergy] in Acute Coronary Syndrome) trial.11
Significant differences were seen both after 30 days and 6 months. After 30 days, the proportion of uncovered stents per strut was 4.3% in the BAS group vs 27.5% in the EES group; the proportion of malapposed struts per strut was 1.2% in the BAS group and 3.2% in the EES group. The differences were maintained at 6 months with 0.8% of uncovered and 0.1% of malapposed struts in the BAS group vs 14.5% of uncovered struts and 1.1% of malapposed struts in the EES group. OCT identified small intrastent thrombi in 2 BAS patients and 7 EES patients at 30 days but in none at 6 months. In addition, neointimal hyperplasia thickness at the strut level was greater in the BAS group (0.25mm) than in the EES group (0.09mm).
The strengths of the present work are the following: a) It uses a randomized study design. b) It uses a sensitive diagnostic method such as OCT to evaluate the study endpoints. c) It included patients with ACS. Previous findings suggest that OCT indicators of delayed healing are more frequently present in patients with ACS than in those with chronic coronary syndromes.14 Therefore, the findings of the present study are relevant to a subset of patients in whom differences in stent characteristics might be more critical than in other clinical scenarios. d) Selection of the assessment time point at 6 months in one of the cohorts is reasonable considering the kinetics of polymer absorption of EES.15e) It compares 2 stent technologies that are specifically designed to address delayed healing by avoiding the presence of permanent polymers.
However, the study also has some limitations. a) Strong evidence in support of a prognostic role of late uncovered/malapposed struts is lacking16 and there is still a need for specifically designed prospective studies with a sufficiently large number of patients. b) The number of patients is limited. c) An OCT assessment at the end of the index procedure is missing. The strut malapposition identified at 30 days and 6 months might have been residual (already present in the acute OCT) or acquired over time. Late acquired malapposition is considered a marker of chronic inflammation and impaired healing and has a more negative perceived impact, although this has not yet been confirmed in clinical studies.17d) Qualitative characterization of the neointima at 6 months is missing in the present study. This might have been more important for the BAS group, which had more abundant neointimal hyperplasia. The characteristics of the neointimal tissue are also ascribed a prognostic role.18
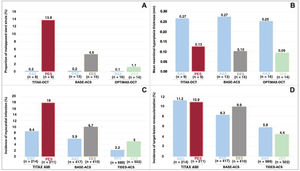
In short, the present OCT study showed greater neointima formation on the one hand and fewer uncovered and malapposed struts on the other hand with BAS compared with a conventional, new-generation DES. Nothing spectacular so far. With a non-DES such as the BAS, this constellation of findings is not surprising. The relevance of the present findings is, however, better assessed if placed in the context of available OCT and clinical evidence from randomized studies on BAS (either Titan-2 or OPTIMAX). There are 3 randomized comparisons of BAS against DES, for which both OCT and clinical outcomes are available (figure 1). In a subset of 18 patients from the TITAX AMI (Titanium Nitride Oxide Coated Stents versus Paclitaxel-Eluting Stents for Acute Myocardial Infarction) trial, OCT was performed at a mean of ∼4 years after randomization. The proportion of uncovered stents at the strut level was 0.4% in the BAS group and 10.8% in the paclitaxel-eluting stent (PES) group. Accordingly, the proportion of malapposed struts was lower in the BAS group (0.2% vs 13.8% in the PES group). Neointimal hyperplasia thickness was 0.27mm in the BAS group and 0.13mm in the PES group.19 The full TITAX AMI trial, which included 425 patients with acute myocardial infarction, showed 5-year outcomes favoring BAS in terms of cardiac death (but not all-cause death), recurrent myocardial infarction and definite stent thrombosis compared with PES, without difference in target lesion revascularization.10 In a subset of 28 patients from the BASE-ACS (Comparison of Bio-Active-Stent to the Everolimus-Eluting Stent in Acute Coronary Syndrome) trial, OCT was performed at a mean of ∼10 months after randomization. The proportions of uncovered and malapposed struts were 0.6% and 0.2%, respectively, in the BAS group and 10.8% and 4.6%, respectively, in the permanent-polymer based EES group. Neointimal hyperplasia thickness was 0.27mm in the BAS group and 0.10mm in the EES group.20 The full BASE-ACS trial, which included 827 patients with ACS showed 5-year outcomes favoring BAS in terms of nonfatal myocardial infarction, while all other outcomes were comparable between BAS and EES, including target lesion revascularization.21 The results of the present OCT study13 should also be considered in relation to the large TIDES-ACS clinical trial, which randomly assigned 1491 patients to either BAS or EES.11 At 18 months of follow-up, outcomes favored BAS in terms of stent thrombosis and myocardial infarction without significant differences in target lesion revascularization.11 However, caution is certainly needed when interpreting the above-mentioned results of the clinical studies because the significant differences in favor of BAS were observed for outcomes that did not represent the primary endpoint of the trials and lacked the required statistical power. Despite all the above-mentioned limitations, a common denominator of the clinical trials is that BAS, a bare-metal stent with a unique coating, might reduce the number of thrombotic events if used in ACS patients without a clinically relevant trade-off in the risk for reintervention. The corresponding OCT studies indirectly suggest that improved vascular healing with BAS might underlie the potential for clinical benefit with this device. The figure displays a summary of the results of these studies, showing safety measures on the left-hand panels and efficacy measures on the right-hand panels. Combined OCT and clinical studies are needed to determine whether isolated short- and mid-term OCT studies are sufficient to predict the long-term performance of coronary stent technologies and obviate the need for large clinical trials with extended follow-up.
Findings of optical coherence tomography and clinical outcomes in randomized studies on bioactive titanium-nitride-oxide-coated stents vs drug-eluting stents. A: malapposed stent struts. B: neointimal hyperplasia thickness. C: myocardial infarction. D: target lesion revascularization. Optical coherence tomography data were obtained after ∼ 4 years in TITAX-OCT,19 ∼10 months in BASE-ACS,20 6 months in OPTIMAX-OCT.13 Clinical outcomes were assessed at 5 years in TITAX AMI10 and BASE-ACS,21 and 18 months in TIDES-ACS.11 OCT data are shown at strut level. BAS, bioactive titanium-nitride-oxide-coated stent; EES, everolimus-eluting stent; PES, paclitaxel-eluting stent.
No specific funding received.
CONFLICTS OF INTERESTNone.