Magnetic resonance has become a first-line imaging modality in various clinical scenarios. The number of patients with different cardiovascular devices, including cardiac implantable electronic devices, has increased exponentially. Although there have been reports of risks associated with exposure to magnetic resonance in these patients, the clinical evidence now supports the safety of performing these studies under specific conditions and following recommendations to minimize possible risks. This document was written by the Working Group on Cardiac Magnetic Resonance Imaging and Cardiac Computed Tomography of the Spanish Society of Cardiology (SEC-GT CRMTC), the Heart Rhythm Association of the Spanish Society of Cardiology (SEC-Heart Rhythm Association), the Spanish Society of Medical Radiology (SERAM), and the Spanish Society of Cardiothoracic Imaging (SEICAT). The document reviews the clinical evidence available in this field and establishes a series of recommendations so that patients with cardiovascular devices can safely access this diagnostic tool.
Keywords
In recent years, magnetic resonance (MR) has become an essential and first-line imaging technique in many clinical situations. At the same time, the number of patients with a cardiovascular device has grown exponentially. It is thus commonplace in clinical practice to encounter a recipient of one of these devices who requires an MR study.1,2 The probability that a recipient of a cardiac implantable electronic device (CIED) will require an MR study in the first postimplantation year is estimated to be 10%, rising to 75% during the patients’ lifespan.3
Although many of these devices do not show a contraindication to MR, some devices are not safe, are only compatible in certain specific circumstances, or require an assessment before and after the scan. In addition, given that they can lead to lower image quality, the indication for the study must be evaluated in the context of the risk-benefit ratio.
The current consensus document has been produced through the collaboration of the Working Group on Cardiac Magnetic Resonance Imaging and Cardiac Computed Tomography of the Spanish Society of Cardiology (SEC-GT CRMTC), the Heart Rhythm Association of the SEC (SEC-Heart Rhythm Association), the Spanish Society of Medical Radiology (SERAM), and the Spanish Society of Cardiothoracic Imaging (SEICAT). The document reviews the safety of MR studies in patients with CIEDs and other cardiovascular devices and establishes practical recommendations to enable all device recipients to safely access this diagnostic modality. Appendix 1 and appendix 2 respectively show the affiliations of each author and each reviewer by the scientific bodies behind the present document.
DEFINITIONSThe MR technique enables image capture due to the interaction between magnetic fields and the hydrogen nuclei of different tissues. Image generation involves application of 3 types of magnetic fields that have different effects on the human body and surrounding objects and influence the safety of the scans.4,5
- 1.
The static magnetic field (B0). This is the magnetic field of the scanner; it is always active and its intensity is measured in teslas (T). The most commonly used systems are 1.5 and 3 T. The interaction of this field with ferromagnetic elements can make objects move, dislodge, or be attracted to the magnet. However, this risk does not typically affect implanted medical devices because they predominantly comprise nonferromagnetic material.
- 2.
Dynamic gradients causing changes in the magnetic field over time (dB/dt). This concerns gradients that are rapidly activated and inactivated during MR studies. These gradients can induce electrical currents in specific devices in the form of heating, vibration, neuromuscular stimulation, and acoustic noise.
- 3.
Radiofrequency (RF) magnetic fields (B1). These fields are predominantly produced in scans of the thoracic area, the location of the antenna emitting the RF. Part of the energy applied is absorbed by the body (measured by the specific absorption rate in W/kg) and converted into heat, its main biological effect. The specific absorption rate increases with the magnetic field and depends on the sequence used. RF magnetic fields show risk of electromagnetic interference with CIEDs.
Depending on the safety profile of the patient undergoing the MR scan, cardiovascular devices are classified as follows:
- 1.
MR-compatible device. Such devices can always safely undergo MR.
- 2.
MR-conditional device. These devices are safe in an MR environment if a series of considerations are taken into account. In the case of CIEDs, the devices have hardware (minimization of ferromagnetic material and modification of leads) and software modifications that permit the safe performance of an MR study under certain conditions.6
- 3.
Non–MR-conditional device. These devices, due to their design or functioning, cannot be ensured to have optimal safety conditions for the performance of MR studies. CIEDs, for example, can undergo MR with a low incidence of complications, as long as certain precautions are taken with device programming, patient monitoring, and MR characteristics.7,8
It must be remembered that, for the determination of the compatibility of a device or set of devices (system), all of the components must be MR compatible or conditional. In the case of CIEDs, both the components (generator and lead) and their combination (ideally the same brand and combination validated for the MR setting) must be compatible and, if required, the conditions of the magnetic field and specific absorption rate must be met, which depend on each model.9
Although MR studies can be safely conducted in patients with compatible or conditional devices, their presence can affect image quality and, thus, test yield. This will depend on the type of the device, its location, the anatomical area being scanned, and the type of sequence used. This factor must be taken into account in the prior evaluation of patients when an MR study is being planned.
INTERACTION BETWEEN MR AND CARDIOVASCULAR DEVICES. SAFETYInfluence of devices on the MR image (artifacts)MR artifacts are relatively frequent. They are defined as any signal increase or loss that has no anatomical basis or is the consequence of information distortion, addition, or loss, generally that related to the presence of metallic or ferromagnetic material. Although some artifacts are obvious and easily recognizable, others are much more subtle and can lead to interpretation and diagnostic errors.
In general, there are 2 types of artifacts induced by ferromagnetic material:
- 1.
Magnetic susceptibility artifacts. These artifacts are the main cause of the appearance of artifacts and are due to a lack of local homogeneity in the field produced by the presence of ferromagnetic material within the magnet.
- 2.
Foucault current artifacts (also called Eddy current artifacts). These artifacts occur because the RF pulse gradients induce electrical currents in the surrounding metallic material, create undesired intermittent magnetic fields that undermine the homogeneity of the field, and distort the image.
In general, artifacts appear as bands of increased or decreased intensity of the signal surrounding the metallic parts. Given that they deteriorate the resonance image, they must be recognized, because they can be confused with a pathological image.10 The magnitude of the artifacts will depend on both the intrinsic characteristics of the device (metallic composition, size, and orientation with respect to the direction of the magnetic field) and its distance from the anatomical region being studied (infrequent in studies outside the thoracic area), the sequence used, and the intensity of the magnetic field.
Influence of MR on devicesIn general terms, the cardiovascular devices with the greatest influence/interference in MR studies are circulatory assist and monitoring devices (left ventricular assist device, extracorporeal membrane oxygenation systems, and continuous cardiac output catheters) and CIEDs (pacemakers, implantable cardioverter-defibrillators [ICDs], cardiac resynchronization therapy devices, and abandoned leads). The former represent an absolute contraindication. In the case of CIEDs, complications are rare but potentially severe.2,11 Due to their higher frequency in the clinical context, table 1 details the potential effects of the MR environment on CIEDs.
Potential effects of the magnetic resonance environment on cardiac implantable electronic devices.
| Effects of MR on CIEDs | Explanation |
|---|---|
| Heating and lesion of the surrounding tissue | Leads can act as antennas for electromagnetic energy and generate currents within the system that increase the temperature of the surrounding tissue, which can damage the local myocardium, elevate the capture threshold, reduce the amplitude of the sensed wave, and, theoretically, increase the threshold for ICD defibrillationIn in vitro studies, the temperature increase is greater for damaged leads, abandoned leads (when there is no connection to a generator acting as a heatsink), and/or epicardial leads (given the absence of convection cooling because it is a space without blood flow). The situation is potentially worse for abandoned epicardial leads |
| Displacement | The magnetic field can displace the ferromagnetic material of the generator, but this is exceptionally rare. MR-compatible devices reduce this risk due to their lower quantity of ferromagnetic materialMost manufacturers advise waiting 6 months after implantation (due to scarring-related “fixation” of the lead), although one series of patients underwent MR in the first few postimplantation days without complications, which is why MR could be early if clinically necessary12 |
| Asynchronous pacing | RF pulses of MR can pass through the lead and stimulate the tissue and induce atrial and/or ventricular arrhythmias |
| Oversensing | RF pulses can generate “noise” (oversensing) that inhibits the pacemaker impulse (risk of asystole in dependent patients) or triggers inappropriate antitachycardia ICD shocks (risk of inappropriate therapies) |
| Magnetically activated reed switch | When the device comes close to a magnet, the device normally paces in asynchronous mode and the antitachycardia therapies of the ICD are inhibited. This behavior is modified in compatible CIEDs when the compatibility mode is activated |
| Electrical reset | The electromagnetic interference generated by MR triggers an electrical reset of the CIED to a manufacturer- and model-specific programming. It generally activates VVI mode (risk of asystole in dependent patients) and antitachycardia therapies can be activated, depending on the nominal diagnostic and therapeutic parameters, typically in a single region of ventricular fibrillation with high-energy discharges (risk of inappropriate therapies) |
| Abnormal battery drainage | Rarely, battery exhaustion can occur; this is more frequent in CIEDs that are nearing the end-of-life replacement of the generator (low battery levels).13 It is a major complication that leads to CIED generator replacement |
CIED, cardiac implantable electronic device; ICD, implantable cardioverter-defibrillator; MR, magnetic resonance; RF, radiofrequency.
In recent years, numerous studies have shown that cardiothoracic and noncardiothoracic MR scans are safe in patients with a CIED as long as a series of measures are taken.2,11
The studies are largely homogeneous in their design (description of the possible adverse effects, device programming, sequences used) and outcomes and concordant and found no clinically significant complications when the studies were performed in 1.5- and 3-T systems according to the defined safety guidelines.14,15Table 2 shows a summary of the main studies in this field.
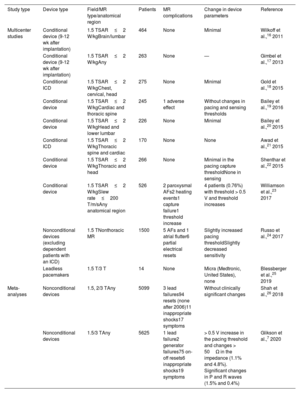
Main multicenter clinical trials and meta-analyses evaluating the safety of magnetic resonance studies in patients with different cardiac implantable electronic devices.
| Study type | Device type | Field/MR type/anatomical region | Patients | MR complications | Change in device parameters | Reference |
|---|---|---|---|---|---|---|
| Multicenter studies | Conditional device (9-12 wk after implantation) | 1.5 TSAR≤2 W/kgBrain/lumbar | 464 | None | Minimal | Wilkoff et al.,16 2011 |
| Conditional device (9-12 wk after implantation) | 1.5 TSAR≤2 W/kgAny | 263 | None | — | Gimbel et al.,17 2013 | |
| Conditional ICD | 1.5 TSAR≤2 W/kgChest, cervical, head | 275 | None | Minimal | Gold et al.,18 2015 | |
| Conditional device | 1.5 TSAR≤2 W/kgCardiac and thoracic spine | 245 | 1 adverse effect | Without changes in pacing and sensing thresholds | Bailey et al.,19 2016 | |
| Conditional device | 1.5 TSAR≤2 W/kgHead and lower lumbar | 226 | None | Minimal | Bailey et al.,20 2015 | |
| Conditional ICD | 1.5 TSAR≤2 W/kgThoracic spine and cardiac | 170 | None | None | Awad et al.,21 2015 | |
| Conditional device | 1.5 TSAR≤2 W/kgThoracic and head | 266 | None | Minimal in the pacing capture thresholdNone in sensing | Shenthar et al.,22 2015 | |
| Conditional device | 1.5 TSAR≤2 W/kgSlew rate≤200 T/m/sAny anatomical region | 526 | 2 paroxysmal AFs2 heating events1 capture failure1 threshold increase | 4 patients (0.76%) with threshold > 0.5 V and threshold increases | Williamson et al.,23 2017 | |
| Nonconditional devices (excluding dependent patients with an ICD) | 1.5 TNonthoracic MR | 1500 | 5 AFs and 1 atrial flutter6 partial electrical resets | Slightly increased pacing thresholdSlightly decreased sensitivity | Russo et al.,24 2017 | |
| Leadless pacemakers | 1.5 T/3 T | 14 | None | Micra (Medtronic, United States), none | Blessberger et al.,25 2019 | |
| Meta-analyses | Nonconditional devices | 1.5, 2/3 TAny | 5099 | 3 lead failures94 resets (none after 2006)11 inappropriate shocks17 symptoms | Without clinically significant changes | Shah et al.,26 2018 |
| Nonconditional devices | 1.5/3 TAny | 5625 | 1 lead failure2 generator failures75 on-off resets6 inappropriate shocks19 symptoms | > 0.5 V increase in the pacing threshold and changes > 50Ω in the impedance (1.1% and 4.8%). Significant changes in P and R waves (1.5% and 0.4%) | Glikson et al.,7 2020 |
AF, atrial fibrillation; ICD, implantable cardioverter-defibrillator; MR, magnetic resonance; SAR, specific absorption rate.
Epicardial and abandoned leads have a higher risk of heating during MR scans. The available evidence in this field is very slight (and nonexistent in the case of epicardial leads) and MR is thus not recommended in these patients and can only be conducted in patients in a severe clinical situation without an alternative diagnostic approach. Nonetheless, a recent publication, with 139 patients and 243 abandoned leads, supported the safety of MR in this clinical situation.8 However, in the case of temporary pacemakers, the characteristics of the generator and lead show a higher risk of complications, which is why they should not be subjected to this type of scan under any circumstance, even though no pertinent clinical evidence is available.12
Regarding leadless pacing devices, although less experience has been accumulated, the initial work has failed to identify a higher rate of complications and they seem safe at both 1.5 and 3 T. Subcutaneous ICDs do not require a distinct approach from that of conventional ICDs, and it must be verified, based on the model and year of manufacture, if they are MR conditional or not.12
Loop recorders are considered safe and do not show any contraindication to scans.27
Other cardiovascular devicesIn the last few years, the numbers and types of cardiovascular and vascular devices that are implanted for the treatment of different conditions have grown exponentially. Although most are safe or conditional in the MR environment, their specific compatibility should be checked. Table 3 lists the most commonly used devices. To summarize, the following can be considered safe, with respect to certain acquisition parameters: coronary and vascular stents, vascular tubes and surgical patches, surgical valvular prostheses (biological and mechanical), transcatheter prostheses and devices (septal occluders, appendage closure devices, and valve replacement and repair), and loop recorders.
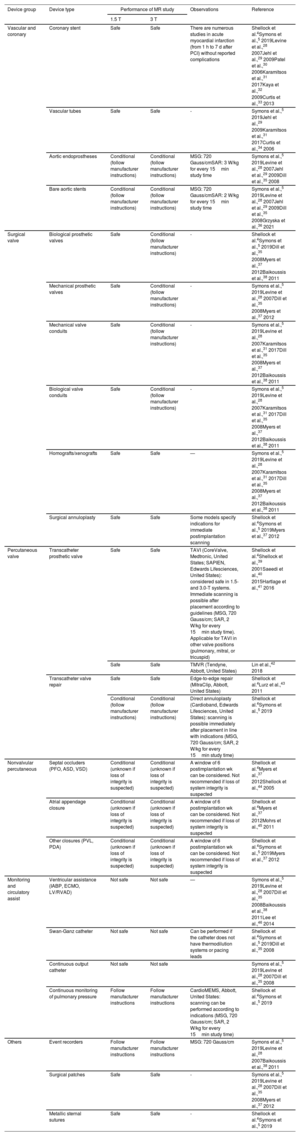
Main types of devices without pacing currently used in cardiology and cardiac surgery: magnetic resonance compatibility and safety in 1.5- and 3-T systems.
| Device group | Device type | Performance of MR study | Observations | Reference | |
|---|---|---|---|---|---|
| 1.5 T | 3 T | ||||
| Vascular and coronary | Coronary stent | Safe | Safe | There are numerous studies in acute myocardial infarction (from 1 h to 7 d after PCI) without reported complications | Shellock et al.4Symons et al.,5 2019Levine et al.,28 2007Jehl et al.,29 2009Patel et al.,30 2006Karamitsos et al.,31 2017Kaya et al.,32 2009Curtis et al.,33 2013 |
| Vascular tubes | Safe | Safe | - | Symons et al.,5 2019Jehl et al.,29 2009Karamitsos et al.,31 2017Curtis et al.,34 2006 | |
| Aortic endoprostheses | Conditional (follow manufacturer instructions) | Conditional (follow manufacturer instructions) | MSG: 720 Gauss/cmSAR: 3 W/kg for every 15min study time | Symons et al.,5 2019Levine et al.,28 2007Jehl et al.,29 2009Dill et al.,35 2008 | |
| Bare aortic stents | Conditional (follow manufacturer instructions) | Conditional (follow manufacturer instructions) | MSG: 720 Gauss/cmSAR: 2 W/kg for every 15min study time | Symons et al.,5 2019Levine et al.,28 2007Jehl et al.,29 2009Dill et al.,35 2008Grzyska et al.,36 2021 | |
| Surgical valve | Biological prosthetic valves | Safe | Conditional (follow manufacturer instructions) | - | Shellock et al.4Symons et al.,5 2019Dill et al.,35 2008Myers et al.,37 2012Baikoussis et al.,38 2011 |
| Mechanical prosthetic valves | Safe | Conditional (follow manufacturer instructions) | - | Symons et al.,5 2019Levine et al.,28 2007Dill et al.,35 2008Myers et al.,37 2012 | |
| Mechanical valve conduits | Safe | Conditional (follow manufacturer instructions) | - | Symons et al.,5 2019Levine et al.,28 2007Karamitsos et al.,31 2017Dill et al.,35 2008Myers et al.,37 2012Baikoussis et al.,38 2011 | |
| Biological valve conduits | Safe | Conditional (follow manufacturer instructions) | - | Symons et al.,5 2019Levine et al.,28 2007Karamitsos et al.,31 2017Dill et al.,35 2008Myers et al.,37 2012Baikoussis et al.,38 2011 | |
| Homografts/xenografts | Safe | Safe | — | Symons et al.,5 2019Levine et al.,28 2007Karamitsos et al.,31 2017Dill et al.,35 2008Myers et al.,37 2012Baikoussis et al.,38 2011 | |
| Surgical annuloplasty | Safe | Safe | Some models specify indications for immediate postimplantation scanning | Shellock et al.4Symons et al.,5 2019Myers et al.,37 2012 | |
| Percutaneous valve | Transcatheter prosthetic valve | Safe | Safe | TAVI (CoreValve, Medtronic, United States; SAPIEN, Edwards Lifesciences, United States): considered safe in 1.5- and 3.0-T systems. Immediate scanning is possible after placement according to guidelines (MSG, 720 Gauss/cm; SAR, 2 W/kg for every 15min study time). Applicable for TAVI in other valve positions (pulmonary, mitral, or tricuspid) | Shellock et al.4Shellock et al.,39 2001Saeedi et al.,40 2015Hartlage et al.,41 2016 |
| Safe | Safe | TMVR (Tendyne, Abbott, United States) | Lin et al.,42 2018 | ||
| Transcatheter valve repair | Safe | Safe | Edge-to-edge repair (MitraClip, Abbott, United States) | Shellock et al.4Lurz et al.,43 2011 | |
| Conditional (follow manufacturer instructions) | Conditional (follow manufacturer instructions) | Direct annuloplasty (Cardioband, Edwards Lifesciences, United States): scanning is possible immediately after placement in line with indications (MSG, 720 Gauss/cm; SAR, 2 W/kg for every 15min study time) | Shellock et al.4Symons et al.,5 2019 | ||
| Nonvalvular percutaneous | Septal occluders (PFO, ASD, VSD) | Conditional (unknown if loss of integrity is suspected) | Conditional (unknown if loss of integrity is suspected) | A window of 6 postimplantation wk can be considered. Not recommended if loss of system integrity is suspected | Shellock et al.4Myers et al.,37 2012Shellock et al.,44 2005 |
| Atrial appendage closure | Conditional (unknown if loss of integrity is suspected) | Conditional (unknown if loss of integrity is suspected) | A window of 6 postimplantation wk can be considered. Not recommended if loss of system integrity is suspected | Shellock et al.4Myers et al.,37 2012Mohrs et al.,45 2011 | |
| Other closures (PVL, PDA) | Conditional (unknown if loss of integrity is suspected) | Conditional (unknown if loss of integrity is suspected) | A window of 6 postimplantation wk can be considered. Not recommended if loss of system integrity is suspected | Shellock et al.4Symons et al.,5 2019Myers et al.,37 2012 | |
| Monitoring and circulatory assist | Ventricular assistance (IABP, ECMO, LV/RVAD) | Not safe | Not safe | — | Symons et al.,5 2019Levine et al.,28 2007Dill et al.,35 2008Baikoussis et al.,38 2011Lee et al.,46 2014 |
| Swan-Ganz catheter | Not safe | Not safe | Can be performed if the catheter does not have thermodilution systems or pacing leads | Shellock et al.4Symons et al.,5 2019Dill et al.,35 2008 | |
| Continuous output catheter | Not safe | Not safe | Symons et al.,5 2019Levine et al.,28 2007Dill et al.,35 2008 | ||
| Continuous monitoring of pulmonary pressure | Follow manufacturer instructions | Follow manufacturer instructions | CardioMEMS, Abbott, United States: scanning can be performed according to indications (MSG, 720 Gauss/cm; SAR, 2 W/kg for every 15min study time) | Shellock et al.4Symons et al.,5 2019 | |
| Others | Event recorders | Follow manufacturer instructions | Follow manufacturer instructions | MSG: 720 Gauss/cm | Symons et al.,5 2019Levine et al.,28 2007Baikoussis et al.,38 2011 |
| Surgical patches | Safe | Safe | - | Symons et al.,5 2019Levine et al.,28 2007Dill et al.,35 2008Myers et al.,37 2012 | |
| Metallic sternal sutures | Safe | Safe | - | Shellock et al.4Symons et al.,5 2019 | |
ASD, atrial septal defect; ECMO, extracorporeal membrane oxygenator; IABP, intra-aortic balloon pump; LV/RVAD, left ventricular/right ventricular assist device; MSG, maximum spatial gradient; PCI, percutaneous coronary intervention; PDA, patent ductus arteriosus; PFO, patent foramen ovale; PVL, paravalvular leak; SAR, specific absorption rate; TAVI, transcatheter aortic valve implantation; TMVR, transcatheter mitral valve repair; VSD, ventricular septal defect.
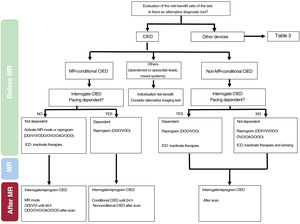
The workflow to consider for patients with cardiovascular devices who are undergoing an MR study will depend on the type of implanted device (figure 1).
This group includes coronary, vascular, and valvular devices. The recommendations can be seen in table 3.
Cardiac implantable electronic devicesThe workflow for the performance of an MR study in patients with implanted CIEDs depends on 3 criteria: a) the condition of the implanted system, including all of its functioning or dysfunctioning annexes; b) the safety conditions of the patients themselves, largely defined by whether they are pacing dependent or not and by the risk of ventricular arrhythmias; and c) the need for an MR study regarding the patients’ clinical condition and the possible existence of alternative diagnostic techniques.
Regardless, prior interrogation of the device is required to assess patients’ pacing dependency and the antibradycardia and antitachycardia programming settings, as well as their subsequent interrogation to confirm their correct functioning and to make the corresponding reprogramming. Figure 1 shows the practical action algorithm in the different contexts.
MR-conditional pacing devicesThe MR compatibility conditions of CIEDs are defined by the manufacturers, which have subjected the complete system to a validation test. However, these tests have been performed using a combination of the different system parts (leads and generator) from the same manufacturer. As specified in the data sheet, the compatibility conditions are only guaranteed under these circumstances; currently and in line with legal requirements, the manufacturers do not guarantee compatibility when materials from different manufacturers are combined, although the relevant literature has shown that this does not increase the risk of complications. Another factor to take into account is the presence of abandoned, dysfunctional, or epicardial material. Under these conditions, no manufacturer guarantees that their systems are MR compatible.
In addition, patients’ pacing dependency is established before the absence of intrinsic or escape rhythms to ensure adequate cardiac output after the cessation of pacing. As a general rule, the European Society of Cardiology (ESC) establishes a threshold of 50 bpm to define patient dependency.9
Qualified staff must assess and revise all patients and their respective systems before the MR scan and after study completion and both actions must be recorded.9 Given that the safety conditions are considered optimal under these circumstances, there is a greater degree of flexibility in the recommended programming modes and the recommended times for the interrogations.
Before the MR, assessment is required of patients (ensuring that they do not have abandoned, epicardial, or dysfunctional leads or extra connectors/adaptors between the lead and generator), as well as interrogation of the device and selection of the MR compatibility mode, if this is one of the programming options of the device being evaluated. Good communication is essential between the MR staff and cardiology to ensure that the system is compatible and that the patient has been correctly evaluated and prepared for the diagnostic examination.
As a general rule, for patients with an intrinsic rhythm, DDI/VVI mode reprogramming is recommended because it allows the patients’ pacing device to function as normal during the scan.9 In addition, this approach permits a longer window for the reprogramming of the device after the MR, with reprogramming recommended within 48hours after the study. As a disadvantage, these programming modes continue to be sensitive to interference from external noise and could trigger the activation of noise reversion algorithms, which give rise to asynchronous autoprogramming modes independent of the baseline heart rate. A possible alternative is reprogramming in ODO/OVO/OAO/OOO modes.9 However, under these conditions, the pacing functionality of the device is lost, which is why reprogramming has to be performed immediately after the MR study.
In the case of pacing-dependent patients with MR-conditional devices without ICD function, an immediate postprocedural revision is not considered necessary but a revision should nonetheless be performed in the first 24hours. The recommended pacing mode under these conditions is DOO/VOO, with a pacing heart rate 20 bpm higher than the intrinsic heart rate, if there is one, or adapted to the hemodynamic needs of the patient if there is no intrinsic rhythm.9
Other programming-related aspects of devices in these circumstances are shown in table 4, in terms of pacing output, sensing parameters, and the additional functions of devices, among others.
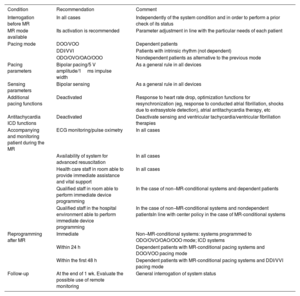
Practical recommendations on the different aspects of safety in patients with cardiac implantable electronic devices undergoing magnetic resonance studies.
| Condition | Recommendation | Comment |
|---|---|---|
| Interrogation before MR | In all cases | Independently of the system condition and in order to perform a prior check of its status |
| MR mode available | Its activation is recommended | Parameter adjustment in line with the particular needs of each patient |
| Pacing mode | DOO/VOO | Dependent patients |
| DDI/VVI | Patients with intrinsic rhythm (not dependent) | |
| ODO/OVO/OAO/OOO | Nondependent patients as alternative to the previous mode | |
| Pacing parameters | Bipolar pacing/5 V amplitude/1ms impulse width | As a general rule in all devices |
| Sensing parameters | Bipolar sensing | As a general rule in all devices |
| Additional pacing functions | Deactivated | Response to heart rate drop, optimization functions for resynchronization (eg, response to conducted atrial fibrillation, shocks due to extrasystole detection), atrial antitachycardia therapy, etc |
| Antitachycardia ICD functions | Deactivated | Deactivate sensing and ventricular tachycardia/ventricular fibrillation therapies |
| Accompanying and monitoring patient during the MR | ECG monitoring/pulse oximetry | In all cases |
| Availability of system for advanced resuscitation | In all cases | |
| Health care staff in room able to provide immediate assistance and vital support | In all cases | |
| Qualified staff in room able to perform immediate device programming | In the case of non–MR-conditional systems and dependent patients | |
| Qualified staff in the hospital environment able to perform immediate device programming | In the case of non–MR-conditional systems and nondependent patientsIn line with center policy in the case of MR-conditional systems | |
| Reprogramming after MR | Immediate | Non–MR-conditional systems: systems programmed to ODO/OVO/OAO/OOO mode; ICD systems |
| Within 24 h | Dependent patients with MR-conditional pacing systems and DOO/VOO pacing mode | |
| Within the first 48 h | Dependent patients with MR-conditional pacing systems and DDI/VVI pacing mode | |
| Follow-up | At the end of 1 wk. Evaluate the possible use of remote monitoring | General interrogation of system status |
ICD, implantable cardioverter-defibrillator; MR, magnetic resonance.
For patients with an MR-conditional ICD, activation of the MR safety mode inactivates ventricular antitachycardia therapies. This situation makes the patient vulnerable to ventricular arrhythmias that could occur before and during the MR study.2
Non–MR-conditional pacing devices or abandoned leadsThis section includes patients with abandoned leads, non–MR-conditional systems (lead and/or generator), epicardial leads, or connectors between the lead and generator.
Studies have shown that, when necessary, MR can be safely performed in such patients with few adverse effects. Both the patient and the physician ordering the MR must be fully cognizant of the type of device and the absence of MR compatibility, the potential risks for the patient, and the possible subsequent dysfunction of the device. Accordingly, the risk-benefit balance must be assessed to determine if the MR scan is required or if it can be replaced by another imaging test.
If, after all of the information is obtained, the MR is considered essential and must be performed, non–MR-conditional pacing devices must be assessed before and immediately after the MR study to ensure their correct functioning. The programming of these devices is summarized in table 4.
Devices with ICD function must also be evaluated and revised before and immediately after the MR study, in this case to reprogram and activate the sensing and antitachycardia therapy functions.2 For ICDs, all antitachycardia therapies must be deactivated, which leaves the patient in a state of vulnerability to ventricular arrhythmias that could occur before and during the MR study.2
In addition, the particular conditions of this group of patients and devices make them vulnerable, which leads to the need for stricter monitoring guidelines and vigilance during MR studies. The general recommendations in this regard, in line with the more recent recommendations of the ESC, are shown in table 4. Regarding the longer-term follow-up, the ESC recommends assessment of system integrity within 1 week. Although there are no literature data supporting the use of remote monitoring for this purpose, it is the opinion of this committee that it represents a clear alternative to an in-person revision.
Risk-benefit balanceThe risk-benefit balance must be assessed in all patients with a clinical indication for MR who have a cardiovascular device. As mentioned, the factors to be considered include the type of device, its compatibility with MR, the patient's pacing dependence, and the clinical need for the MR study, as well as if diagnosis can be reached using an alternative imaging technique.
With the due precautions, the presence of these devices should not be a limitation for MR with an established or urgent clinical indication. In this regard, the MagnaSafe study showed that nonthoracic MR studies with 1.5-T systems are safe in non–MR-conditional ICD or pacemaker recipients if the devices have been appropriately programmed before the scan.24
In general terms, there is more evidence on the compatibility of devices in 1.5-T systems than in MR systems with a stronger magnetic field. Accordingly, and if their use does not result in a study of insufficient diagnostic quality, 1.5-T systems would be preferable.
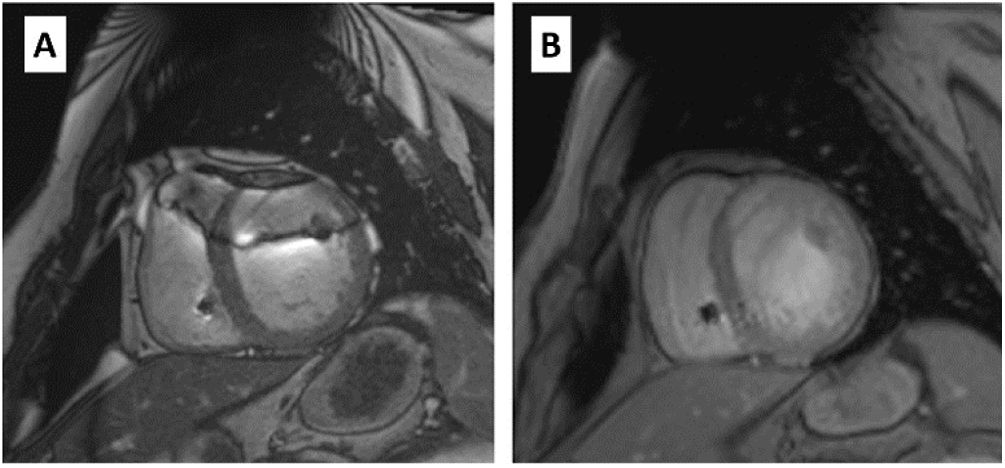
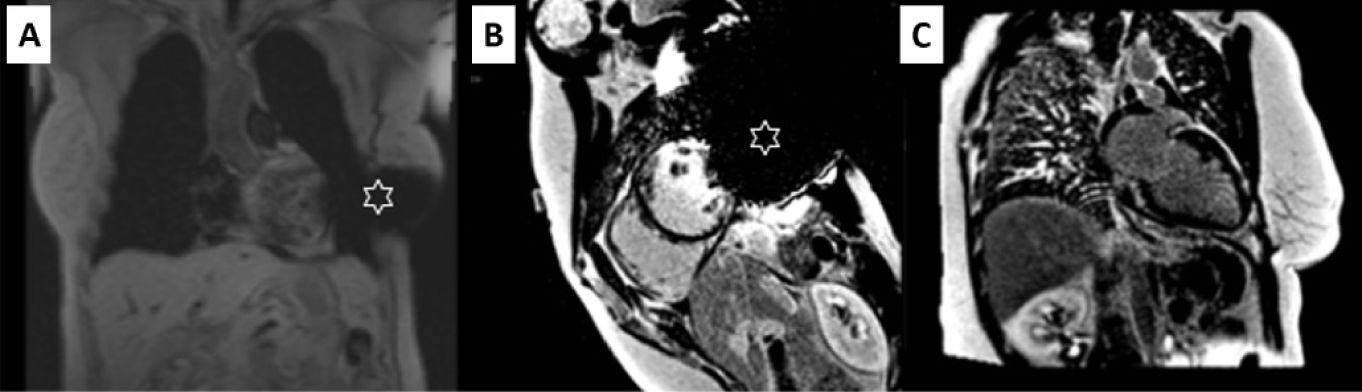
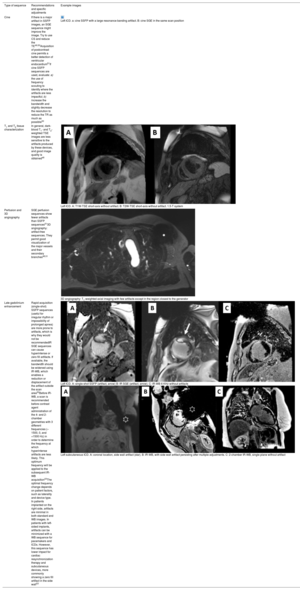
IMPROVEMENT AND OPTIMIZATION OF THE CARDIOTHORACIC MR IMAGING OF PATIENTS WITH DEVICESIn an MR study, the type of device and its location constitute the main determinants of image quality. In cardiothoracic MR, ICDs and cardiac resynchronization therapy usually produce more artifacts due to their larger size and inclusion of ferromagnetic elements and can show image distortion up to 12cm from the generator (figure 2). For this reason, devices implanted on the left side create more artifacts than right-sided devices,47 which generally do not affect cardiac imaging.48
Images obtained with a 1.5-T MR scanner in a patient with a pacemaker. A: in a cine SSFP (steady-state free precession) sequence, artifacts are visible in the thoracic wall (white arrows) that affect the anterior region (asterisk) in the 2-chamber image; however, image measurement in the short-axis is possible. B: in late gadolinium enhancement images (IR SGE [inversion-recovery spoiled gradient echo]), the artifact is less marked and an area of late gadolinium enhancement (black arrow) can be correctly assessed in both geometries.
The cardiac MR scanning protocol must be aimed at answering the clinical question, must be limited to essential sequences, and must be as short as possible (figure 3).
Practical image optimization algorithm for patients with cardiac implantable electronic devices undergoing cardiothoracic MR studies. CRT, cardiac resynchronization therapy; ICD, implantable cardioverter-defibrillator; IR SGE/wideband late gadolinium enhancement, inversion-recovery spoiled gradient echo late gadolinium enhancement; MR, magnetic resonance; SGE, spoiled echo gradient; TSE, turbo spin-echo.
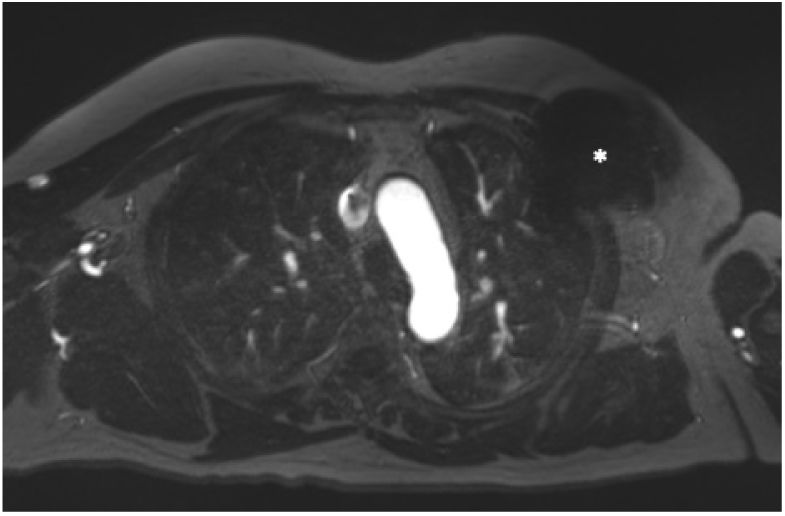
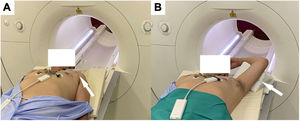
In general, to reduce artifacts secondary to CIEDs, attempts should be made to increase the distance between the device and the scanning area. Some of the useful maneuvers are to place the arm ipsilateral to the generator above the head or to acquire the image with deep inspiration (figure 4).
Preparation of a patient with an automatic implantable device in the left hemithorax for cardiothoracic MR. Increased distance between the generator and the scanning area through placement of an adhesive band (A) or with the arm ipsilateral to the device raised above the head (B).
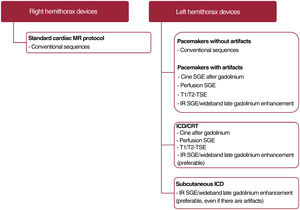
Regarding the selection of protocols and scanning sequences, the recent use must be highlighted of wideband late gadolinium enhancement to minimize the artifacts associated with CIEDs, although these sequences are still not clinically available in many centers. Table 5, figure 3, and figure 4 gather these and other specific recommendations related to the scanning sequences used in cardiac MR studies.
Recommendations for improving cardiothoracic imaging according to the magnetic resonance sequence used.
| Type of sequence | Recommendations and specific adjustments | Example images |
|---|---|---|
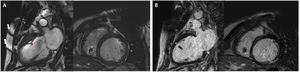
| Cine | If there is a major artifact in SSFP images, an SGE sequence might improve the image. Try to use CS and reduce the TE49,50Acquisition of postcontrast cine permits a better detection of ventricular endocardium51If cine SSFP sequences are used, evaluate: a) the use of frequency scouting to identify where the artifacts are less impactful; b) increase the bandwidth and slightly decrease the resolution to reduce the TR as much as possible50 | Left ICD. a: cine SSFP with a large resonance banding artifact. B: cine SGE in the same scan position |
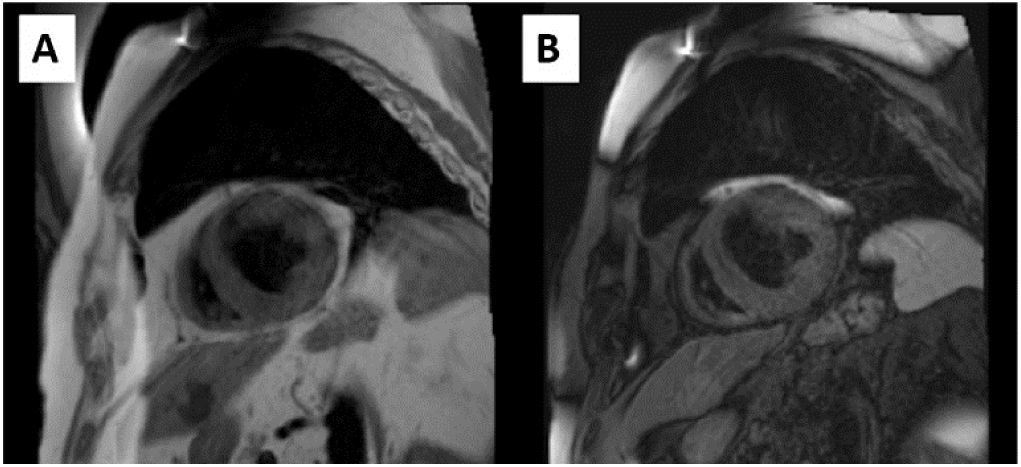
| T1 and T2 tissue characterization | In general, dark-blood T1- and T2-weighted TSE images are less sensitive to the artifacts produced by these devices, and good image quality is obtained48 | Left ICD. A: T1W-TSE short-axis without artifact. B: T2W-TSE short-axis without artifact. 1.5-T system |
| Perfusion and 3D angiography | SGE perfusion sequences show fewer artifacts than SSFP sequences513D angiography: artifact-free sequences. They permit good visualization of the major vessels and their secondary branches48,51 | 3D angiography: T1-weighted axial imaging with few artifacts except in the region closest to the generator |
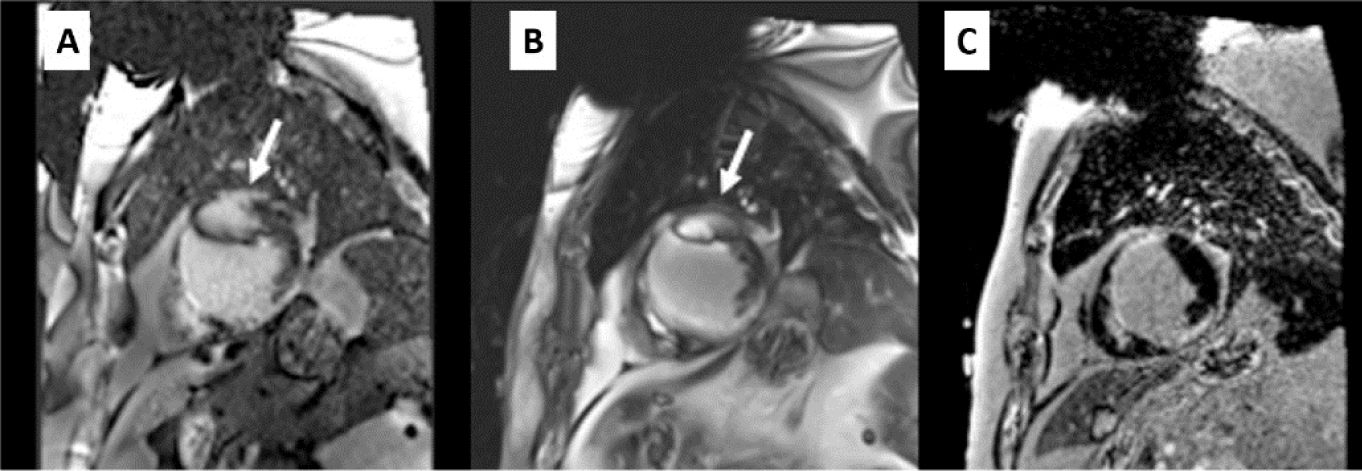
| Late gadolinium enhancement | Rapid acquisition (single-shot) SSFP sequences (useful for irregular rhythm or impossibility of prolonged apnea) are more prone to artifacts, which is why they would not be recommendedIR SGE sequences can cause hyperintense or zero fill artifacts. If available, the bandwidth should be widened using IR-WB, which enables a reduction or displacement of the artifact outside the scan area52Before IR-WB, a scan is recommended before contrast agent administration of the 4- and 2-chamber geometries with 3 different frequencies (–1500, 0, and +1500 Hz) in order to determine the frequency at which hyperintense artifacts are less likely. This optimum frequency will be applied to the subsequent IR-WB acquisition53The optimal frequency change depends on patient factors, such as laterality and device type. In patients implanted on the right side, artifacts are minimal in both standard and WB images. In patients with left-sided implants, artifacts can be minimized with a WB sequence for pacemakers and ICDs. However, this sequence has lower impact for cardiac resynchronization therapy and subcutaneous devices, more commonly showing a zero fill artifact in the side wall53 | Left ICD. A: single-shot SSFP (artifact, arrow) B: IR SGE (artifact, arrow). C: IR-WB 6 KHz without artifactsLeft subcutaneous ICD. A: coronal location, side wall artifact (star). B: IR-WB, with side wall artifact persisting after multiple adjustments. C: 2-chamber IR-WB, single plane without artifact |
3D, 3-dimensional; CS, compressed sensing; ICD, implantable cardioverter-defibrillator; IR, inversion-recovery; SGE, spoiled gradient echo; SSFP, steady-state free precession; TE, echo time; TR, repetition time; TSE, turbo spin-echo; WB, wideband.
MR studies can be performed in patients with a cardiovascular device with the appropriate precautions. The present document reviews the evidence and provides management guidelines to minimize interference of the magnetic field with the electronic device and to reduce the image artifacts generated with cardiovascular devices in the region of the cardiothoracic scan.
FUNDINGNone.
AUTHORS’ CONTRIBUTIONSAll authors have participated equally in the drafting of this document. The present document has been reviewed before publication by experts not involved in its drafting: J.F. Rodríguez Palomares, J.M. Tolosana, J.A. Hidalgo Pérez, E. Pérez-David, Vicente Bertomeu-González, and H. Cuéllar.
CONFLICTS OF INTERESTNone.
Working Group on Cardiac Magnetic Resonance Imaging and Cardiac Computed Tomography of the Spanish Society of Cardiology (SEC-GT CRMTC): M. Barreiro-Pérez, A. Maceira González, S. Prat-González, L.J. Jiménez-Borreguero, and C. Fernández-Golfín Lobán.
Heart Rhythm Association of the Spanish Society of Cardiology (SEC-Heart Rhythm Association): D. Calvo and T. Datino.
Spanish Society of Medical Radiology (SERAM) and the Spanish Society of Cardiothoracic Imaging (SEICAT): B. Cabeza, J.L. Reyes-Juárez, E. Vañó Galván, C. Delgado Sánchez-Gracián, R.J. Perea, G. Bastarrika, and M. Sánchez.
Working Group on Cardiac Magnetic Resonance Imaging and Cardiac Computed Tomography of the Spanish Society of Cardiology (SEC-GT CRMTC): J.F. Rodríguez-Palomares and E. Pérez-David.
Heart Rhythm Association of the Spanish Society of Cardiology (SEC-Heart Rhythm Association): J.M. Tolosana and V. Bertomeu-González.
Spanish Society of Medical Radiology (SERAM) and the Spanish Society of Cardiothoracic Imaging (SEICAT): J.A. Hidalgo Pérez and H. Cuéllar.



![Images obtained with a 1.5-T MR scanner in a patient with a pacemaker. A: in a cine SSFP (steady-state free precession) sequence, artifacts are visible in the thoracic wall (white arrows) that affect the anterior region (asterisk) in the 2-chamber image; however, image measurement in the short-axis is possible. B: in late gadolinium enhancement images (IR SGE [inversion-recovery spoiled gradient echo]), the artifact is less marked and an area of late gadolinium enhancement (black arrow) can be correctly assessed in both geometries. Images obtained with a 1.5-T MR scanner in a patient with a pacemaker. A: in a cine SSFP (steady-state free precession) sequence, artifacts are visible in the thoracic wall (white arrows) that affect the anterior region (asterisk) in the 2-chamber image; however, image measurement in the short-axis is possible. B: in late gadolinium enhancement images (IR SGE [inversion-recovery spoiled gradient echo]), the artifact is less marked and an area of late gadolinium enhancement (black arrow) can be correctly assessed in both geometries.](https://static.elsevier.es/multimedia/18855857/0000007600000003/v2_202401190650/S1885585722003218/v2_202401190650/en/main.assets/thumbnail/gr2.jpeg?xkr=eyJpdiI6IkRqYzNzYS9vRmxFNlpGS3c5djlYcFE9PSIsInZhbHVlIjoiMUR5UkNQNWZYWjY4dHJ4QkQ0b0FodVJkNUlpUU40RHd5aUNKTkVteFRNND0iLCJtYWMiOiI2MTUwOTliZTM5OWRlMmJhYWE0YjBjNGVlNzk4ZDUwYWYxNThkNDYyMDY2Y2NjODViNWVhZjY1MDVkMzk0OGI2IiwidGFnIjoiIn0=)