Residual lipid risk has been defined as the excess of cardiovascular events observed in patients with adequate control of low-density lipoprotein cholesterol and has been mainly attributed to high-density lipoprotein cholesterol and triglycerides. The aim of our study was to describe the clinical features and the magnitude and characteristics associated with residual lipid risk in patients with a history of coronary revascularization.
MethodsMulticenter, observational, cross-sectional study of patients with a history of coronary revascularization. Residual lipid risk was defined as the presence of high-density lipoprotein cholesterol <40 mg/dL and/or triglycerides >150 mg/dL in patients with low-density lipoprotein cholesterol <100 mg/dL.
ResultsWe included 2292 patients with a mean age of 65.5 (12.4) years; 94.1% were receiving no statin therapy and 4.8% no lipid therapy. Statin-only therapy (74%) was the most common strategy, followed by combination with ezetimibe (17%). The prevalence of high-density lipoprotein cholesterol <40 mg/dL was 35.8%, hypertriglyceridemia 38.9%, and low-density lipoprotein cholesterol >100 mg/dL 44.9%; the residual lipid risk group included 29.9% of all patients. This patient group had a similar clinical profile except for slightly lower mean age, higher incidence of diabetes, and higher proportion of men. Multivariate analysis identified positive associations of diabetes and male sex with residual lipid risk; current smoking, male sex, and fibrate therapy were associated with high-density lipoprotein cholesterol <40 mg/dL; current smoking, abdominal obesity, and fibrate therapy were associated with hypertriglyceridemia.
ConclusionsIn daily clinical practice, almost one-third of patients with a history of coronary revascularization have low-density lipoprotein cholesterol <100 mg/dL plus low high-density lipoprotein cholesterol and/or hypertriglyceridemia, a concept known as residual lipid risk.
Keywords
Hypercholesterolemia is a known independent risk factor for ischemic heart disease.1, 2, 3 Serum low-density lipoprotein (LDL) concentration has a direct linear correlation with the incidence of cardiovascular complications, particularly ischemic events; in fact, the target of hypercholesterolemia control is serum LDL cholesterol (LDLc) concentration.4, 5 Lipid metabolism is highly complex, however, and various lipoproteins involved in the onset, development, and destabilization of the entire atherosclerotic process have been identified, mainly high-density lipoproteins (HDL),6, 7, 8, 9 triglycerides,10, 11, 12 and very-low-density lipoproteins (VLDL).13 The excess of complications observed despite close control of cardiovascular risk factors has been called “residual risk” and is attributed to the coexistence of other risk factors, the progressive nature of atherothrombotic disease, and with regard to residual lipid risk, other lipid abnormalities in patients with controlled LDLc, particularly low HDL cholesterol (HDLc) and/or high triglycerides.4, 14
The true prevalence of residual lipid risk is not well described, but it appears to be more prevalent in patients with proven cardiovascular disease, particularly ischemic heart disease.14, 15, 16 Secondary prevention studies have focused on describing the degree of serum LDLc concentration control,17, 18, 19, 20, 21 and there are few data on other lipid profile abnormalities in patients with ischemic heart disease.
The latest theories on atherosclerosis propose that there is an imbalance (more apolipoprotein B-rich lipoproteins, such as LDL, chylomicrons, or VLDL, than apolipoprotein A-rich lipoproteins) in atherosclerosis formation and destabilization.9, 22 However, these determinations are not usually available in daily clinical practice and serum LDLc, HDLc, and triglyceride concentrations are the determinations used in most clinical settings involved in cardiovascular prevention. The determinations, which would partly explain what is known as “residual risk,” are useful and have predictive value, and most scientific evidence is based on these measurements.1, 12, 23, 24 Additionally, statins are the main therapeutic approach to hypercholesterolemia, particularly in patients with proven ischemic heart disease, and have an extremely potent effect on LDL, but little to none on HDL or triglycerides.25
The hypothesis of the present study is that patients with ischemic heart disease would have a prevalence of residual lipid risk despite a high rate of lipid-lowering therapy and, therefore, the purposes of the study were to analyze the clinical characteristics and magnitude of residual risk in a population of patients with ischemic heart disease (defined as a history of surgical or percutaneous coronary revascularization) and to describe the clinical features associated with the presence of residual risk.
METHODS Study DesignThe study was designed as a multicenter, observational, cross-sectional analysis of patients with a history of percutaneous coronary intervention (PCI) or surgical coronary revascularization (bypass). The inclusion criteria were age older than 18 years, surgical or percutaneous coronary revascularization more than 3 months earlier, complete medical history available, consent to participate in the study, and signing of the informed consent. The only exclusion criteria were refusal to provide informed consent or failure to meet any of the inclusion criteria. Patients with a history of revascularization more than 3 months previously were enrolled for the purpose of including stable patients able to perform their daily activities and maintain their usual diet. In total, 205 cardiology investigators throughout Spain who were listed in the scientific sections of the Spanish Society of Cardiology (Sociedad Española de Cardiología) were invited to participate, 199 of whom finally participated. The investigators belonged to 16 autonomous communities and recruited patients from outpatient clinics linked to a hospital (162; 81.4%) or at specialist outpatient clinics (37; 18.6%). Each investigator included the first 14 consecutive patients seen at the outpatient clinic who met the inclusion criteria. A total of 2460 patients were recruited; 168 were excluded because they did not meet all study criteria, including availability of all data needed for the analysis. The final analysis included data from 2292 (93.2%) patients.
A study-specific paper questionnaire was prepared for each patient. Age, weight, height, waist circumference, cardiologic history, and medical treatments and doses were recorded for all patients. The study protocol and informed consent were approved by the Ethics Committee of the Complejo Hospitalario Universitario Clínico de Santiago de Compostela, A Coruña, Spain.
To establish the sample size necessary for the study purposes, a nonprobabilistic random sampling method (inclusion of patients in strict consecutive order) was used. If maximum uncertainty (p=q=50%), a precision level of 2%, and 95% confidence interval (CI) are assumed, then the sample size needed would be 2377 assessable patients. To ensure the quality and proper conduct of the study, an outside company (Phidea S.L.) was contracted, and the study centralized all information produced during the study to ensure the accuracy of the data collected. A database was regenerated and directly sent to the scientific committee for the study.
Variable DefinitionPatients who achieved the following serum values were considered to meet the therapeutic targets for lipid control: total cholesterol <180mg/dL; LDLc <100mg/dL; HDLc >40mg/dL, and triglycerides <150mg/dL4; therefore, residual lipid risk was defined as patients with LDLc control (<100mg/dL) but without control of HDLc (<40mg/dL) and/or triglycerides (>150mg/dL). Patients were considered to have a history of diabetes mellitus if a previous diagnosis was recorded in the medical history, the patient followed specific drug therapy, or 2 consecutive fasting blood glucose measurements were above 126 mg/dL.26 Hypertension was defined as 2 consecutive blood pressure measurements ≥140/90mmHg or specific antihypertensive therapy.27 Body mass index >30 was classified as obesity, and a waist circumference >102 cm in men or >88 cm in women was considered abdominal obesity.4 Glomerular filtration rate was estimated from serum creatinine concentrations according to the abbreviated formula of the Modification of Diet in Renal Disease study28: 186×creatinine – 1.154×age – 0.203 (× 0.742 in women).
A history of gait claudication, revascularization of lower limbs, amputation, or established diagnosis was coded as peripheral arterial disease. A history of stroke was defined as a diagnosis of ischemic, hemorrhagic, or transient stroke recorded in the medical history or in a medical report. All patients were asked about their alcohol intake; patients who reported regular consumption of some kind of alcoholic beverage were classified according to alcohol intake of 1-2 glasses of wine/day, 1-2 beers/day, 3-4 alcoholic beverages/week, or in case of higher quantities, alcohol intake was specifically recorded in g/day. The degree of physical activity was classified as sedentary (no episode/event >30 min/wk), moderate (<3 weekly episodes of exercise >30 min), or vigorous (>3 weekly episodes of exercise >30 min).
Statistical AnalysisAll variables followed a normal distribution and are expressed as the mean (standard deviation), except for triglycerides which is shown as median [interquartile range]. The mean values of qualitative variables were compared by the χ2 test and the quantitative variables were compared using the Student t test. The multivariate analysis was carried out using binary logistic regression, and the results were expressed as age- and sex-adjusted odds ratio (OR) (95% CI). Significance was set at a P value of ≤.05. All data were processed using the SPSS 15.0 statistical package (SPSS Inc., Chicago, Illinois, United States).
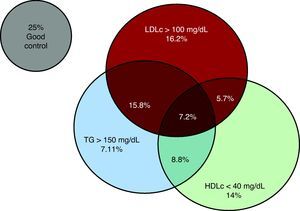
RESULTSA high proportion of the 2292 patients included had some lipid profile abnormality: 44.9% had LDLc >100mg/dL, 38.5% had HDLc <40mg/dL, and 38.9% had triglycerides >150mg/dL. When the combination of these lipid profile abnormalities was analyzed, 29.9% presented HDLc <40mg/dL and/or triglycerides >150mg/dL with LDLc levels <100mg/dL (Figure 1); these patients comprised the residual lipid risk group. This patient subgroup had a slightly lower mean age, and a higher prevalence of diabetes mellitus, male sex, and PCI coronary revascularization (Table 1). No other relevant differences in laboratory test results apart from the parameters included in the definition of residual lipid risk were observed (Table 2).
Figure 1. Combination of the various lipid profile abnormalities. HDLc, high-density lipoproteins cholesterol; LDLc, low-density lipoproteins cholesterol; TG, triglycerides.
Table 1. General Characteristics of Patients According to Type of Presence of Residual Lipid Risk.
| Total | No RLR | RLR | P | |
| Patients | 2292 | 1606 (70.1) | 686 (29.9) | |
| Age, y | 65.5 (12.4) | 66.1 (12.2) | 64.1 (12.7) | <.01 |
| Men, % | 78.2 | 76.5 | 82 | <.01 |
| History, y | 3.3 (4.2) | 3.4 (4.3) | 3 (3.9) | .03 |
| PCI, % | 76.6 | 74.6 | 81.2 | <.01 |
| Systolic BP, mmHg | 132.3 (18.6) | 133.1 (18.5) | 130.3 (18.6) | .01 |
| Diastolic BP, mmHg | 75.9 (10.9) | 76.2 (10.9) | 75.1 (10.9) | .02 |
| BMI | 28.5 (4.1) | 28.4 (4.1) | 28.5 (4) | .54 |
| Excess weight, % | 49.3 | 50.4 | 46.8 | .12 |
| Obesity, % | 29.1 | 28.3 | 30.9 | .21 |
| Waist circumference, cm | 98.8 (13.3) | 98.7 (13.5) | 99.1 (12.6) | .47 |
| Abdominal obesity, % | 44.3 | 43.9 | 45 | .66 |
| Dyslipidemia, % | 71.7 | 72.7 | 69.3 | .16 |
| Diabetes mellitus, % | 33.2 | 31.6 | 37 | .01 |
| Hypertension, % | 60.8 | 61.1 | 60.1 | .65 |
| Smokers, % | 48.8 | 47.5 | 51.8 | .06 |
| Ex-smokers, % | 10.4 | 9.8 | 11.8 | .16 |
| Sedentary lifestyle, % | 42.4 | 43 | 41.1 | .41 |
| Alcohol consumption, % | 28.6 | 29.1 | 27.4 | .42 |
| GFR <60 mL/min/1.72 m2, % | 22.8 | 22.8 | 22.6 | .41 |
| Peripheral arterial disease, % | 11.4 | 11.5 | 11.3 | .88 |
| History of stroke, % | 7.2 | 7.3 | 7 | .84 |
| Other CVD, % | 6.2 | 5.7 | 7.5 | .12 |
BMI, body mass index; BP, blood pressure; CVD, cardiovascular disease; GFR, glomerular filtration rate; PCI, percutaneous cutaneous intervention; RLR, residual lipid risk.
The data are expressed as no. (%) or mean (standard deviation) unless otherwise indicated.
Table 2. Results of Biochemical Determinations Based on the Presence of Residual Lipid Risk.
| Total | No RLR | RLR | P | |
| Total cholesterol, mg/dL | 169.4 (40.2) | 181.2 (39.6) | 144.3 (27.9) | <.01 |
| LDL, mg/dL | 95.6 (33.5) | 106.7 (34.2) | 73.8 (17.5) | <.01 |
| HDL, mg/dL | 46 (16.7) | 49.5 (15) | 39 (17.9) | .14 |
| Triglycerides, mg/dL | 122 [93-167] | 112 [88-149.8] | 151 [106.2-188.8] | <.01 |
| Blood glucose, mg/dL | 123.8 (3.9) | 122.5 (44.2) | 126.5 (43.2) | .12 |
| Blood glucose in diabetics, mg/dL | 143.4 (48.4) | 142.4 (50) | 154.4 (45.3) | .42 |
| HbA1C in diabetics, % | 7.5 (6.2) | 7.6 (7) | 7.4 (4.5) | .73 |
| Creatinine, mg/dL | 1.1 (0.5) | 1 (0.4) | 1.2 (0.7) | .04 |
| GFR, mL/min/1.72 m2 | 76.9 (27.4) | 77 (28.7) | 76.7 (24.4) | .8 |
| ALT, IU/L | 31 (22.1) | 30.6 (19.1) | 31.7 (27.7) | .33 |
| AST, IU/L | 29.4 (20.3) | 30 (22) | 28.1 (15.8) | .07 |
| GGT, IU/L | 48.7 (54.8) | 49.1 (53.4) | 47.8 (57.9) | .66 |
| Creatine kinase, mg/dL | 103.7 (76.3) | 103.7 (69.7) | 103.8 (89.1) | .97 |
| C-reactive protein, mg/L | 16.9 (56.6) | 16 (51) | 19.3 (69.6) | .64 |
ALT, alanine transaminase; AST, aspartate transaminase; GFR, glomerular filtration rate; GGT, gamma-glutamyl transaminases; HbA1c, glycohemoglobin; HDL, high-density lipoproteins; LDL, low-density lipoproteins; RLR, residual lipid risk.
All values are shown as mean standard deviation, except for triglycerides which are shown as median [interquartile range].
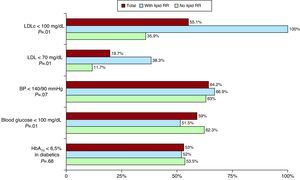
Regarding lipid-lowering therapy, patients at residual lipid risk were most commonly treated with atorvastatin and less often with ezetimibe, and no significant differences were observed in the use of the remaining lipid-lowering agents or their doses (Table 3). Patients with residual lipid risk presented better control of serum LDLc concentration, but poorer control of baseline blood sugar; there were no significant differences in blood pressure or glycohemoglobin control (Figure 2).
Table 3. Use of Lipid-Lowering Agents (%) and Dose Used (Mean [Standard Deviation]) According to Presence of Residual Lipid Risk.
| Total | No RLR | RLR | P | |
| Atorvastatin | 57.8 | 56.1 | 61.7 | .01 |
| Dose, mg/dL | 40 (21.8) | 39.5 (22) | 41.2 (21.4) | .18 |
| Simvastatin | 22.7 | 23 | 22 | .61 |
| Dose, mg/dL | 27.4 (11.9) | 27.4 (12.3) | 27.3 (11.1) | .88 |
| Pravastatin | 6.2 | 6.4 | 6 | .73 |
| Dose, mg/dL | 28.9 (10.7) | 28.7 (10.6) | 29.5 (11.2) | .7 |
| Lovastatin | 0.4 | 0.4 | 0.6 | .49 |
| Dose, mg/dL | 30 (19.4) | 33.3 (24.2) | 25 (10) | .54 |
| Fluvastatin | 6.4 | 6.9 | 5.1 | .1 |
| Dose, mg/dL | 75.5 (14.5) | 74.9 (15.7) | 77.7 (9.6) | .33 |
| Ezetimibe | 18.3 | 19.7 | 15 | <.01 |
| Fibrates | 3.7 | 3.2 | 4.8 | .06 |
RLR, residual lipid risk.
Figure 2. Control of risk factors according to the presence of residual lipid risk. BP, blood pressure; HbA1c, glycohemoglobin; LDLc, low-density lipoproteins cholesterol; RLR, residual lipid risk.
Table 4 contains the results of the multivariate analysis of characteristics associated with the presence of low HDLc, high triglycerides, or residual lipid risk. Diabetes was the only variable, along with lower age, that associated the 3 abnormalities; male sex was also associated with the presence of residual lipid risk, whereas age and ezetimibe therapy were negatively associated. However, diabetes, active smoking, male sex, and fibrate therapy were associated with low HDLc, whereas diabetes, smoking, abdominal obesity, and fibrate therapy were associated were hypertriglyceridemia.
Table 4. Multivariate Analysis of the Factors Associated With the Presence of High-Density Lipoprotein Cholesterol, High Triglycerides, or Any of These 2 Abnormalities in the Presence of Low-Density Lipoprotein Cholesterol <100mg/dL (Residual Lipid Risk).
| Variables | HDL<40 mg/dL | P | Triglycerides>150 mg/dL | P | RLR | P |
| Age | 0.98 (0.98-0.99) | <.01 | 0.98 (0.98-0.99) | <.01 | 0.99 (0.98-0.99) | .02 |
| Male sex | 1.81 (1.39-2.34) | <.01 | 1.14 (0.89-1.46) | .3 | 1.52 (1.16-1.98) | <.01 |
| Smoking | 1.57 (1.14-2.15) | <.01 | 1.39 (1.01-1.92) | .04 | 1.18 (0.85-1.63) | .33 |
| Diabetes mellitus | 1.32 (1.07-1.63) | <.01 | 1.46 (1.19-1.8) | <.01 | 1.35 (1.09-1.68) | <.01 |
| BMI>30 | 1.11 (0.87-1.41) | .4 | 1.22 (0.96-1.54) | .1 | 1.09 (0.85-1.4) | .48 |
| Abdominal obesity | 1.07 (0.85-1.34) | .59 | 1.33 (1.06-1.68) | .01 | 1.07 (0.84-1.36) | .61 |
| Sedentary lifestyle | 1.14 (0.93-1.4) | .22 | 1.06 (0.87-1.31) | .55 | 0.91 (0.73-1.13) | .38 |
| PCI | 1.18 (0.93-1.49) | .18 | 1.26 (1-1.6) | .05 | 1.4 (1.09-1.8) | <.01 |
| Ezetimibe | 0.99 (0.97-1.02) | .44 | 1.02 (0.99-1.04) | .19 | 0.97 (0.94-0.99) | <.01 |
| Fibrates | 1.25 (1.13-1.38) | <.01 | 1.39 (1.24-1.57) | <.01 | 1.04 (0.94-1.15) | .43 |
BMI, body mass index; HDLc, high-density lipoprotein cholesterol; LDLc, low-density lipoprotein cholesterol; PCI, percutaneous cutaneous intervention; RLR, residual lipid risk.
The results are shown as odds ratio (95% confidence interval).
The results of this broad cross-sectional study conducted in Spain indicate that, despite statin therapy in almost all patients who had undergone myocardial revascularization, only one-fourth of patients achieve the lipid targets recommended by the current clinical practice guidelines, and most have 1 or more criteria for dyslipidemia. In addition to observing that a high proportion of patients did not reach the LDLc target, a considerable proportion of cases had low HDLc and high triglyceride concentrations both in the presence of controlled and noncontrolled LDLc. These facts highlight the importance of the need to improve the combined lipid profile, particularly in this patient group at high coronary risk.
Despite a number of cross-sectional epidemiologic studies that have investigated the prevalence of dyslipidemia in various populations with different levels of cardiovascular risk,29 in diabetics,17, 30 and in patients with clinical cardiovascular disease,16, 17, 18, 20, 21, 31 this is the first study in Spain to analyze the characteristics of a large cohort of patients who had undergone percutaneous or surgical coronary revascularization. Additionally, previous studies usually focused on LDLc, without a more complete analysis of patients’ lipid profile,18, 20 except for a recent DYSIS substudy of patients enrolled in Spain.21 In this regard, we observed that a high proportion of patients do not achieve the LDLc targets or present a relevant residual lipid risk and also that the control of other risk factors is similar in these patients. It is true, however, that the group of patients with residual lipid risk is much more homogeneous than the group of patients without residual risk, which, although it includes patients with noncontrolled LDL (>100 mg/dL), also includes some with excellent lipid control (LDLc <100 mg/dL, HDLc>40 mg/dL, and triglycerides <150 mg/dL). Our study was intended to show the prevalence of residual lipid risk and its associated clinical characteristics and stresses the importance of undertaking efforts to improve the entire lipid profile, particularly in high-risk patients.
Coronary revascularization –particularly PCI revascularization– has become a widespread clinical practice used in both stable and unstable patients,32, 33 making PCI the most common procedure among patients with ischemic heart disease. Patients with stable chronic ischemic heart disease are at high risk of long-term complications, and their risk factors should be carefully monitored. According to data from the last European Action on Secondary and Primary Prevention by Intervention to Reduce Events (EUROASPIRE),34 dyslipidemia is the risk factor most closely controlled in the past decade, with almost 55% of patients with on-target LDLc, data that are consistent with our results. Other secondary prevention registers in Spain16, 18, 20, 21 show similar results, although there is a paucity of data on residual lipid risk, low HDLc, and/or high triglycerides in patients with LDLc <100mg/dL. Only 1 substudy of patients enrolled in Spain in the DYSIS study analyzed these aspects and had similar findings in patients with ischemic heart disease, even though the study included primary and secondary prevention patients.21 This effect appears to be particularly common and relevant in patients with ischemic heart disease, as observed in the third National Health and Nutrition Examination Survey (NHANES), which found that only 20.3% of subjects with ischemic heart disease had good control of LDLc, HDLc, and triglycerides, compared to 66.7% of subjects without the disease.15
Statin therapy rates in patients with ischemic heart disease have increased exponentially in recent decades,34 although these patients continue to present a high cardiovascular risk partly attributable to persistent lipid profile abnormalities. Interest in the role of low HDLc concentrations is growing, due to evidence regarding its implications in all phases of atherosclerosis22; however, the actual contribution of hypertriglyceridemia to cardiovascular risk has been subject to considerable debate and is not widely accepted.35 It is true that serum HDLc concentrations tend to be low when triglyceride levels are high: they are related to similar liver and lipid metabolisms, particularly influenced by the presence of obesity,36 diabetes,7, 16, 37 or some other components of metabolic syndrome.38 Data from secondary prevention studies, such as Treatment to New Targets7 and Incremental Decrease in End Points through Aggressive Lipid Lowering,11 clearly show the prognostic value of HDL as residual risk despite treatment with maximum statin doses; however, a primary prevention substudy of the JUPITER study did not observe this effect.39 Conversely, in a sample of Spanish workers, the triglyceride-to-HDL ratio was the best predictor of the incidence of a first myocardial infarction.24 These data reflect that the concept of residual lipid risk may have a relevant role in cardiovascular prevention, although more data are needed to accept this fully and to include it in clinical practice.
As mentioned, most previous studies on dyslipidemia in patients with ischemic heart disease have focused on LDLc based on the National Cholesterol Educational Program recommendations (NCEP-ATPIII).4 Our study includes a broader perspective and also considers HDLc and triglyceride concentrations as well as LDLc concentrations. In fact, the results of our study indicate a high frequency of more than 1 lipid profile abnormality; in the aggregate group, 37.5% of patients showed 2 or 3 simultaneous lipid abnormalities. Abnormal HDLc and/or triglyceride levels were observed in 1 of every 3 patients, a fact that represents a significant therapeutic challenge as well as a significant residual risk associated with severe dyslipidemia. The presence of other risk factors and comorbidities such as diabetes and ischemic heart disease can also substantially increase patients’ total cardiovascular risk. An inverse association between the plasma HDL concentration and the incidence of ischemic heart disease has been reported.6, 8, 37 The PROCAM cohort confirms this association even after adjustment for other cardiovascular risk factors.8 Although various analyses have found a linear relationship between triglyceride levels and the incidence of ischemic heart disease, this relationship is not maintained after a multivariate analysis; this could be due to considerable biological and analytical interindividual and intraindividual variability, but also to the high prevalence of additional HDLc and LDLc abnormalities. However, according to a recent consensus conference on management of dyslipidemia in patients at high cardiometabolic risk, therapy to raise HDLc and to lower triglycerides is a Class II indication.40
Based on a multivariate model used to assess the potential determinants of lipid control, we identified several variables associated with 1 or more lipid abnormalities. Both age and ezetimibe therapy showed a negative association with presentation of any of the abnormalities implied in residual lipid risk. The presence of diabetes showed a significant correlation with poor control of HDLc and triglycerides. These findings are not uncommon in diabetics treated with statins who present relatively low levels of LDLc but usually present abnormal HDLc and triglyceride levels.15, 16
The main limitations of the ICP-Bypass register are related to the study design. First of all, because this is a cross-sectional study, it was not possible to define risk factors or the risk of subsequent cardiovascular complications, only clinical associations. Secondly, because these patients had stable ischemic heart disease, the results cannot be extrapolated to patients with acute or unstable coronary syndrome, although the previous lower treatment of patients at the onset of their coronary disease leads us to believe that the residual risk could be even greater in these patients. Thirdly, lipid concentrations were not analyzed in a central laboratory. Lastly, because patients were only included if their entire medical history was available, it is possible that the patients selected had closer medical follow-up and therefore better control of risk factors and serum cholesterol concentrations. Likewise, because patients were only included by cardiologists, patients with better control of their risk factors may have been selected, as there was also no monitoring to ensure that investigators were enrolling consecutive patients.
CONCLUSIONSOne-third of patients with a history of coronary revascularization and LDLc <100mg/dL present low HDLc and/or high triglycerides, and therefore fall into the category of residual lipid risk. Diabetes and younger lower age are the main characteristics associated with the presence of residual lipid risk. The prevalence of residual risk affects one-third of all patients with a history of revascularization.
FUNDINGThe ICP-Bypass registry was funded by an unrestricted grant from MSD España.
CONFLICTS OF INTERESTNone declared.
Received 20 December 2010
Accepted 24 May 2011
Corresponding author: Servicio de Cardiología, Hospital Universitario de Santiago de Compostela, Travesía de la Choupana s/n, 15706 Santiago de Compostela, A Coruña, Spain. jose.Ramon.Gonzalez.Juanatey@sergas.es