Keywords
INTRODUCTION
The clinical perspective of non-ST segment elevation acute coronary syndromes (NSTE ACS) has changed greatly in recent years. The clinical concept itself has only recently been coined as a result, above all, of a better understanding of the pathophysiological processes ACS have in common. The first clinical practice guidelines on NSTE ACS management were not published until 20001-3 and were quickly revised in the light of various studies4-6 that added information of particular relevance to therapy. At the same time, a joint committee of the European Society of Cardiology and the American College of Cardiology7 proposed a controversial change in the definition of acute myocardial infarction (AMI) that was received with no little skepticism by Spanish cardiologists.8
The Spanish Society of Cardiology (SEC) Section for Ischemic Heart Disease perceived that the haste and magnitude of changes to the definition and to recommendations for NSTE ACS patient management might make appropriate, homogeneous medical attention more difficult. A further difficulty was the fact that the numerous specialists who participate in the diagnosis and treatment of NSTE ACS patients (emergency room physicians, cardiologists, intensivists, internists, etc) do not always apply homogeneous criteria. However, despite these peculiarities of clinical care and the fact that NSTE ACS constitutes the clinical presentation of ischemic heart disease that causes most urgent hospital admissions, little information is available about the clinical care of these patients in Spain.
To evaluate the reality of this situation, the SEC Section for Ischemic Heart Disease and Coronary Units initiated a project to describe the state of ACS in Spain thru a registry given the acronym DESCARTES. This article describes the characteristics, management and prognosis of these patients.
PATIENTS AND METHODS
Design
This prospective, observational, cohort study aimed to register all consecutive NSTE ACS patients hospitalized during April 2002 in a representative sample of Spanish hospitals, both public and private, accustomed to managing these patients.
Selection of Centers (Figure 1)
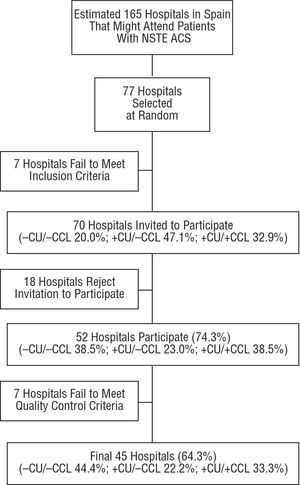
Figure 1. Flow chart showing process of selection, acceptance, and exclusion of centers and its influence on center type representativeness. CU indicates coronary care unit; CCL, cardiac catheterization laboratory; NSTE ACS, non-ST segment elevation acute coronary syndrome.
From Spanish Ministry of Health records, we compiled a list of ≥50 bed, non-specialist centers (i.e. hospitals specializing in pediatrics, trauma, obstetrics, etc were excluded). Using this list, we conducted a telephone survey to identify centers that received ≥5 NSTE ACS admissions/month. From centers that met this criterion, we made a random selection classifying centers in 3 groups according to the cardiological facilities available: a) centers with cardiology service, coronary unit (CU) or intensive care unit (ICU), and cardiac catheterization laboratory (CCL) where coronary interventions are performed; b) centers with CU or ICU, without CCL; and c) centers without cardiology service, CU or ICU, or CCL. The number of centers in each group was proportional to the volume of patients treated. In addition, according to the average number of admissions for each hospital type, we estimated that they admitted >50, 20-50, or 5-19 NSTE ACS patients/month, respectively, in each group.
From these estimates, we calculated that to recruit 2000 patients in 1 month we would need to invite 66 hospitals to participate: 22 primary, 36 secondary, and 19 tertiary care centers. The projected sample size was set at some 1500 patients in order to achieve ±0.025 precision in observed proportions of 50% (maximum standard deviation). We over-selected by 25% to compensate for possible sample loss following quality control and increased the hospital sample by 10%-20% to compensate for centers failing to meet the minimum admissions criterion. Initially, 77 centers were invited to participate, selected at random from the list of Spanish hospitals routinely admitting ACS patients. Seven centers failed to meet minimum ±5 ACS admissions/month criterion and declined the invitation, most of which were centers with CU or ICU, but without CCL. Of the rest, 52 (74.3%) agreed to participate, although a further 7 were later excluded for failure to fulfill quality control criteria. Final participation was 64.3% and all Spanish regions were represented except Murcia, in the south-east, and La Rioja, in the north, omitted due to the random selection procedure.
Patients
The registry included all patients hospitalized in participating hospitals for >24 hours with symptoms that could be diagnosed as NSTE ACS, unless they had complete blockage of the left branch of the bundle of His-Purkinje.
Enrolment
In the month programmed for the study, April 2002, patient enrolment was lower than expected. Consequently, we extended enrolment into May, during which 39 centers contributed.
Permission
The study was approved by at least 1 ethical committee. Patients were asked to provide written informed consent before inclusion in the registry.
Variables and Data Collection
We analyzed patients' clinical, electrocardiographic, and laboratory characteristics as well as their clinical evolution in-hospital, treatment received and service or department of admission. Computer software was designed specifically for data collection with automatic encryption, compression and e-mail transmission direct to the company responsible for data management. Results were submitted continuously by each center; cases were considered closed when data on all variables had been entered on discharge.
Definitions
Definitions of variables can be found at www.scisquemica.net/PROY/descartes/descartes2.htm, the web page of the SEC Section for Ischemic Heart Disease.
Follow-up
Centralized telephone follow-up at 6 months retrospectively evaluated the appearance of major cardiovascular events: a) death (cause of death), and b) rehospitalization (cause of rehospitalization, time elapsed following the onset of each event).
Quality Control
We used an opportunist methodology based on the concentration of hospitals in large urban areas with monitors who were external to these centers. We selected 16 (30%) of the 52 centers (in Barcelona, Madrid, Valencia, and Zaragoza) and verified 10 key variables (age, gender, diagnosis on discharge, Q wave AMI, service or department of admission, electrocardiogram [ECG] on admission, diabetes, hypertension, hypercholesterolemia, smoking). Centers were warned there would be quality control without specifying which hospitals or how many patients would be involved. In these 16 hospitals, quality control was carried out on all patients if total enrolment in the center amounted to <40 patients. In centers recruiting ≥40 patients, we selected 40 patients per center at random. Kappa index of agreement was 0.7-1 (average, 0.9). Each center provided a list of discharges during the study period and we calculated the rate of coverage (number of ACS enrolled *100/number of ACS discharged) with a minimum 70% limit. Copies of the ECGs that qualified patients for inclusion and of ECGs performed prior to discharge were analyzed centrally.
Statistical Analysis
Continuous variables are expressed as mean ± standard deviation (SD) or as median and range if they did not fit a normal distribution. Categorical variables are given as percentages of valid responses. We constructed Kaplan-Meier survival curves and compared these using the Mantel-Cox test. Statistical analysis was performed with SPSS 11.5.
RESULTS
Between 1 April and 31 May 2002 we enrolled 2017 patients in 52 participating centers. Results from 7 centers were excluded for failure to meet established quality control criteria leaving 1877 patients. Patient numbers by hospital type were: 436 (23.2%) from the 20 centers without CU/ICU or CCL; 344 (18.3%) from the 10 centers with CU/ICU and without CCL, and 1097 (58.4%) from the 15 hospitals with CU/ICU and CCL.
Clinical characteristics of the population are in Table 1 and clinical episodes motivating admission in Table 2. Table 3 shows diagnostic and therapeutic procedures used in-hospital. During hospitalization, 94.6% of patients received antiplatelet agents and 81% heparin (of which 88% received low molecular weight heparin). Coronary angiography was performed on 41% of patients; 26% of procedures took place in the first 48 hours; significant coronary heart disease was found in 81% of studies (multivessel coronary disease in 52%, disease of the left coronary artery in 9%). Coronary revascularization, usually percutaneous procedures, was performed on 56% of patients undergoing coronary angiography.
Table 4 shows in-hospital evolution. Fifty-one patients died in-hospital (2.7%), 39 from cardiac, 2 from cerebrovascular, and 10 from other causes, including 2 unknown. Most patients received a definitive diagnosis of unstable angina (54%) or non-Q wave AMI (25%); following admission, symptoms in 18% of patients were diagnosed as of non-coronary origin. Treatment on discharge for all patients is shown in Table 4. If we exclude patients discharged with a diagnosis of chest pain of non-coronary origin, on discharge 91% were prescribed antiplatelet agents (82% aspirin, 37% clopidogrel, and 1.6% ticlopidine), 60% beta-blockers, 59% statins, and 43% angiotensin converting enzyme (ACE) inhibitors.
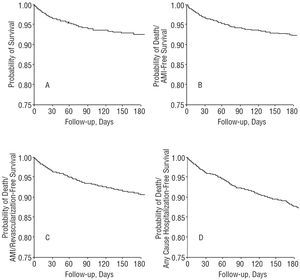
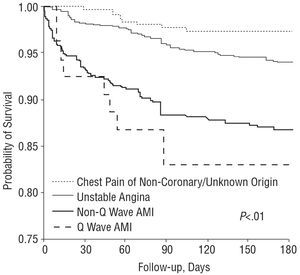
A complete 6 month follow-up was possible in 1762 patients (93.9%) (Table 4 and Figure 2). As suggested by the different diagnoses on discharge, 6-month mortality was highest in patients with Q wave and non-Q wave AMI (Figure 3). Differences between all possible pairs of curves were statistically significant (P<.01 in all comparisons).
Figure 2. Survival curves at 6 months in 1877 DESCARTES patients for events: A. Death. B. Death or hospitalization for acute myocardial infarction. C. Death, hospitalization for acute myocardial infarction or coronary revascularization. D. Death or hospitalization for any cause. AMI indicates acute myocardial infarction.
Figure 3. Six-month survival curves in 1877 DESCARTES patients in terms of diagnosis on discharge. Mantel-Cox test P<.01 for comparison of any pair of curves. AMI indicates acute myocardial infarction.
DISCUSSION
The DESCARTES study is the first representative Spanish registry of routine clinical practice in NSTE ACS patients. The method, based on random selection proportional to hospital type defined in terms of cardiological facilities and data collection quality control, gives great reliability when results are extrapolated to the day-to-day reality of care. The DESCARTES study shows that although NSTE ACS patients in Spain have a high-risk cardiovascular profile only a quarter are admitted to intensive care units. Moreover, patients receive suboptimal treatment and present high 6-month morbidity and mortality.
The population studied is of relatively advanced age, slightly above that reported in other European registries (64-66 years),9-15 and has a very high prevalence of risk factors, especially diabetes mellitus which is greater than in other registries.9-13,15 Similarly, a high proportion of patients presented a family history of cardiovascular illness, principally chest angina, and was receiving cardiovascular drug treatment. However, although almost 3 out of 4 patients were diagnosed with cardiovascular disease, fewer than half received aspirin; moreover, 27% of patients received beta-blockers when more than half had a history of angina and 29% had a history of infarction. Similarly, statin and ACE inhibitor use prior to the event was lower than would correspond to the risk level of the population studied.
In the initial ECG that led to hospitalization, 76% of patients presented anomalies in repolarization although ST-segment deviation was only found in 40%. Initial diagnosis was unstable angina in 70% and AMI in the remaining 30%. However, in discharge reports 18% were diagnosed with chest pain of non-coronary origin. These patients presented 2.6% 6-month mortality which suggests some possible diagnostic inaccuracy. Notably, although 35% presented ST-segment depression in initial ECGs and half showed some degree of myocardial necrosis marker elevation, only 1 in 4 patients with suspected NSTE ACS was admitted to an intensive care coronary unit. This is proportionately fewer than in the Italian registry (which, moreover, only included patients with unstable angina)9 or in the EuroHeart Survey,15 which reported approximately half of the patients were admitted to intensive care coronary units.
From the pharmacologic point of view, the high use of oral antiplatelet agents and heparin, generally low molecular weight heparin, stand out. We cannot draw conclusions on the 37% use of clopidogrel as its clinical indication was in transition. In the 2000 guidelines, use of clopidogrel was not contemplated except in patients contraindicated for aspirin or when associated with coronary stent implantation1. However, the CURE study4 may well have influenced hospitals. Use of glycoprotein IIb/IIIa inhibitors was low and may have been indicated for coronary angioplasty rather than NSTE ACS; this remains unexplained although a similar low rate is reported elsewhere.12,13 Spanish guidelines on NSTE ACS in 2000,1 unlike Spanish, European, or North American2,3 guidelines on antithrombotic treatment,16 described results of studies into glycoprotein IIb/IIIa inhibitors without establishing a level of recommendation and this may have influenced their use. It would be of interest to evaluate glycoprotein IIb/IIIa inhibitor use following publication of the new recommendations. In-hospital use of beta-blockers, ACE inhibitors or statins (drugs proven to reduce mortality in NSTE ACS patients) was excessively low: 40%-60%. Beta-blockers and ACE inhibitor use was below that reported elsewhere.13-15
Althou gh global use of troponins was high, the proportion of patients with troponin elevation was higher than in those finally diagnosed with AMI. The study design does not permit an explanation of this discrepancy but it would be consonant with the skepticism and/or rejection by numerous Spanish cardiologists of the new definition of AMI reported in the SEC Section for Ischemic Heart Disease survey conducted following publication of the definition.8
In a quarter of the population, total cholesterol levels were not determined during hospitalization. Although almost half the patients were discharged without an echocardiographic study, this proportion is greater than in other registries.9,15 Perhaps, this is because many patients had a prior history of cardiovascular illness and, in particular, of myocardial infarction or coronary revascularization, obviating the need for echocardiography in patients whose systolic function was already known. Non-invasive tests for ischemia were performed on 40% of patients which is more than described by other authors9,15 and may be due to the less selective enrolment procedure in DESCARTES. Cardiac catheterization was performed on 41% of patients, which is similar to the figure described in all but the older registries9-12; percutaneous coronary revascularization was performed on just over half of patients, which is similar to that reported in other registries.10-13 It should be pointed out that almost 10% of patients undergoing coronary angiography had significant stenosis of the left coronary artery and more than 25% had stenosis of 3 coronary arteries, despite which only 4.2% of all patients underwent surgical coronary revascularization during initial hospitalization and another 3.3% were rehospitalized for revascularization during follow-up.
Representativeness of Results
One of the most important aspects of this study is the representativeness of the results. The only previous large-scale Spanish registry on NSTE ACS, the project for the study of prognosis in angina (known by the acronym PEPA), recruited patients from those initially admitted to 18 centers selected nationally on the single criterion of having a CCL and entailed a probable bias in attitude to diagnosis and therapy.10,11 The DESCARTES study uses a random, stratified selection of participating centers (based on availability of principal cardiological resources). This, together with a 67% participation of the centers selected, confers substantial external validity on results. In addition, the quality control, with 6.9% exclusions, ensures internal validity. Among the most recent European registries of NSTE ACS patients (Table 5),9-15 only PRAIS-UK (recruiting 1046 patients in 56 British centers during 9 months in 1998) used a similar method12. The largest non-Spanish registries, such as EuroHeart and GRACE,14,15 have involved volunteer centers, making the generalization of results less reliable. The DESCARTES results can be interpreted as a reliable approximation to routine clinical care of NSTE ACS patients in Spain.
Implications of the Study
From a reasonably representative sample of Spanish clinical reality, DESCARTES has shown deficiencies in the care of NSTE ACS patients. This finding should be a stimulus to revise the clinical care of these patients and initiate qualitative improvements adapted to the clinical recommendations that, in this case, were updated a few months after the completion of the study.17,18 Therefore, new registries of activity should be constructed to enable us to determine whether deficiencies in care detected by DESCARTES persist or have been reduced. Similarly, the extent to which latest clinical practice guidelines are met should be determined and as should the impact of their fulfillment on patient prognosis. The errors and limitations of DESCARTES, such as overestimation in the capacity for recruitment and the decision to prolong the enrolment period, the rate of loss participating centers classified according to cardiological facilities available due to failure to meet admission or quality control criteria, or the rate of patient loss during follow-up, can facilitate improved research design and the development of similar studies in the future.
CONCLUSIONS
The DESCARTES study results show that although patients admitted to Spanish hospitals with suspected NSTE ACS have a high incidence of complications both in-hospital and in 6-month follow-up, some efficacious diagnostic and therapeutic measures are used less than current clinical practice guidelines recommend. Data indicate that substantial opportunities exist to improve the quality of medical care of patients with NSTE ACS in Spain.
ANNEXE. Participating Centers, Principal Researchers, and Contribution to the Study
Hospital Clínico San Carlos, Madrid: A. Fernández-Ortiz (203); Hospital General Universitario Gregorio Marañón, Madrid: H. Bueno (127); Hospital Clínico Universitario Lozano Blesa, Zaragoza: F. Roncalés (121); Ciutat Sanitaria de Bellvitge, L'Hospitalet de Llobregat: E. Esplugas (110); Hospital Clínico Universitario de Valencia, Valencia: L. Fácila (96); Hospital Provincial de Navarra, Pamplona: N. Basterra (92); Hospital Miguel Servet, Zaragoza: F.J. Monzón (92); Hospital Costa del Sol, Marbella: E. González (84); Hospital San Agustín, Avilés: V.M. Rodríguez (73); Hospital de la Santa Creu i Sant Pau, Barcelona: J. García (63); Complejo Hospitalario Virgen de la Victoria, Málaga: E. de Teresa (54); Hospital Marina Baixa de Villajoyosa, Villajoyosa: I. Antorrena (49); Hospital Ramón y Cajal, Madrid: E. Asín (42); Hospital Clínic, Barcelona: M. Heras (40); Complejo Hospitalario Alarcos, Ciudad Real: L. Ruiz-Valdepeñas (37); Hospital de Palamós, Palamós: A. Gómez (37); Hospital Universitario de Girona Josep Trueta, Girona: J. Sala (36); Hospital General Yagüe, Burgos: A.J. Montón (34); Hospital Verge de la Cinta, Tortosa: Ll. Gutiérrez (32); Hospital Universitario Príncipe de Asturias, Alcalá de Henares: A. Cambronero (31); Hospital del Mar, Barcelona: J. Bruguera (31); Hospital San Eloy, Barakaldo: J.A. Novales (31); Hospital Joan XXIII, Tarragona: A. Bardají (26); Hospital Nuestra Señora de Arantzazu, Donostia: P. Marco (25); Hospital Can Misses, Ibiza: J. Seguí (25); Hospital de l'Esperit Sant, Santa Coloma de Gramanet: T. Poble (22); Hospital Comarcal de la Axarquia, Vélez: J. Zafra (22); Hospital de Sant Jaume, Calella: A. Aloy (22); Hospital de Terrassa, Terrassa: M.D. Martínez (21); Hospital Comarcal de La Selva, Blanes: A. Zamora (17); Hospital Virgen del Camino, San Lúcar de Barrameda: A. Revello (17); Hospital Comarcal Sierrallana, Torrelavega: B. Gutiérrez (16); Hospital la Inmaculada, Huercal-Overa: E. Morillo (15); Hospital de Sant Pau i Santa Tecla, Tarragona: J.C. Soriano (15); Clínica Juaneda, Palma de Mallorca: J. Soler (15); Hospital General del INSALUD, Soria: J. Martínez (12); Hospital Virgen del Puerto, Plasencia: A. Sáez (12); Clínica Santa María de la Asunción, S.A., Tolosa: J.R. Beramendi (10); Policlínico Vigo, S.A. (POVISA), Vigo: F. Noriega (10); Hospital General de Requena, Requena: L. Mainar (10); Hospital Comarcal Monforte de Lemos, Monforte de Lemos: M.L. Vázquez (9); Hospital Princesa de España, Jaén: O. Cuadra (9); Hospital José María Díaz Domínguez, Minas de Riotinto: L. García (9); Hospital de la Línea, La Línea de la Concepción: E. Rueda (9); Clínica Quirón, Barcelona: J. Riba (5); Institut Municipal d'Investicació Mèdica, Barcelona: J. Marrugat, S. Tello, and H. Martí.
The annex includes a list of researchers and hospitals participating in the DESCARTES study. The DESCARTES study was initiated by the Section for Ischemic Heart Disease and Coronary Units of the Spanish Society of Cardiology in collaboration with Bristol-Myers Squibb thru the award of an unconditional grant.
Correspondence: Dr. H. Bueno.
Servicio de Cardiología. Hospital General Universitario Gregorio Marañón.
Dr. Esquerdo, 46. 28007 Madrid. España.
E-mail: hecbueno@jet.es