The article published in Revista Española de Cardiología describing the 10-year follow-up after alcohol septal ablation (ASA) in patients with hypertrophic obstructive cardiomyopathy (HOCM) is a welcome addition to the literature.1 Although the series reflects an earlier experience with a technique that continues to evolve over time, the encouraging long-term outcomes bode well for the next generation of patients who will undergo ASA after what we would hope is an adequate trial of medical therapy. The numbers of patients in this reported experience from 5 centers is small, as are the numbers undergoing surgical myectomy. It would have been of interest to know whether the use of septal reduction therapy increased over time. In addition, it would have been interesting to know whether the surgical myectomy population differed from those undergoing ASA or whether the decision was made on the basis of institutional expertise and facilities.
The authors clearly state in their discussion that the results of either septal myectomy or ASA depend on operator experience, as well as an understanding of the disease itself. We and others have shown that the success and complications rate of ASA significantly improve after a procedural volume of >50 patients.2 Thus, it would be expected that the long-term results of ASA in Spain will become even better as centers gain greater experience. There is a balance between a higher volume of alcohol used in the procedure producing better hemodynamic results and the possible long-term detrimental consequences of adverse myocardial remodeling and ventricular arrhythmias. The mean of 3.7mL of alcohol used in the study herein discussed is larger than the 1.5 to 2.0mL used by more experienced centers and may account for the need for transplant and implantable cardioverter-defibrillators (ICD) in the patient cohort. The selection of smaller branches of septal perforator arteries that directly target the region of systolic anterior motion with septal contact using echographic contrast injections allows for smaller volumes of alcohol while maintaining the optimal hemodynamic results. Other changes in technique, such as using small, flexible, screw-in temporary pacemaker wires to avoid myocardial perforation, all come with experience.
The patient population in the study herein discussed appears to be an ideal cohort of patients to undergo ASA. They are primarily older women, who would have an increased risk of septal myectomy. Interestingly, 42% of the patients had received a pacemaker prior to the ASA procedure, which mitigates against the worrisome—and most common—complication of complete heart block, which can occur hours to days after ASA. Based upon these results, the number of ASA in this particular population can be expected to increase in Spain.
In a recent interview, Dr. Braunwald stated that “in looking back on my career, the work on hypertrophic cardiomyopathy was clearly the most exciting that I have ever done”.3 Hypertrophic cardiomyopathy is a young disease and, although the first anatomic description of diffuse muscular hypertrophy of the left ventricular outflow tract was published in 1907,4 the first contemporary description was reported in the 1950s5 and, as our understanding of this disease or group of disease entities has grown, it remains a topic of interest and fascination. The last 60 years have witnessed major advances in our understanding of the natural history of this disease, the role of obstruction, and other mechanisms including diastolic function, the unraveling of the web of molecular genetics, and different therapeutic approaches.
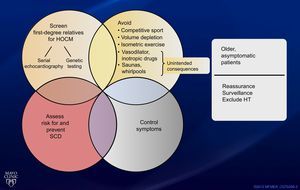
Figure 1 illustrates the key aspects of patient management. This editorial will focus on approaches to controlling symptoms in patients with obstruction, which is the subject of the paper by De la Torre Hernández et al,1 but the screening of first-degree relatives for HOCM and the risk stratification for sudden cardiac death and its prevention for an ICD are nonetheless crucial aspects of patient management.6 Irrespective of symptomatic status, patients with HOCM should avoid competitive sport, volume depletion, vasodilator and inotropic drugs, and isometric exercise and should exercise care in using saunas and whirlpools. Low-intensity aerobic exercise as part of a healthy lifestyle should be encouraged, as should control of risk factors that may contribute to cardiovascular disease.6
The emphasis on surgical and percutaneous approaches to septal reduction therapy should not detract from the fact that most patients referred for an initial evaluation will be symptomatically controlled pharmacologically.7 The goals of medical therapy are to reduce the exercise-induced gradient and myocardial oxygen demand and to prolong the diastolic filling period. The cornerstones of therapy are beta-blockers alone or in combination with verapamil and diltiazem, while disopyramide use is more variable. These drugs have been tried and tested and have been around for approximately 50 years, and their mechanisms of benefit are logical.3,6 Nonetheless, it is sobering to note the relative paucity of objective data on the efficacy of pharmacologic therapy. Spoladore7 reviewed the literature from 1950 to 2011 and identified 45 studies involving only 2121 patients, of which 40 studies were prospective, only 7 studies included 50 or more patients, and there were only 5 randomized controlled trials. Nonetheless, clinical experience is favorable and the proof of the pudding is in the eating, in the sense that clinical success is measured by an improvement in symptoms and by the absence of adverse drug effects.
After a long hiatus, there appears to be a resurgence of interest in identifying potential new therapeutic targets in HOCM. These include aldosterone and angiotensin II antagonists to prevent myocardial fibrosis,7 perhexiline, which is a metabolic modulator that alters myocardial energetics and improves intracellular metabolic efficacy,8 and ranolazine, a potent inhibitor of the late sodium current, which in turn may affect abnormal energy handling and arrhythmogenicity.7 Other agents, including ivabradine, ranolazine, and cyclic guanosine monophosphate activators, may theoretically be helpful in targeting myocardial subendocardial ischemia. The extent to which the pharmacologic management of HOCM will be changed by these new agents remains to be determined.
SURGICAL SEPTAL MYECTOMYA corrective operation to relieve outflow tract obstruction was first described in 1961 by Morrow at the National Institute of Health and by Kirklin and Ellis at the Mayo Clinic.4 Nonetheless, in the early years the operative risk was substantial, and surgery was limited to only a few centers. Moreover, this was a time when the entity of true obstruction remained a source of debate.4,9,10 A major stimulus, however, to the use of surgical and percutaneous approaches to septal reduction therapy came about when Maron et al11 demonstrated that left ventricular outflow tract gradients occur with rest or during exercise in almost 70% of patients, and that this was associated with symptoms and adverse clinical outcomes.
Over the years, transaortic surgical myectomy has become a safer procedure, and mortality in expert centers is less than 1%. The procedure has also become more extensive, and in the outflow tract excision is more extensive and frequently includes resection of the midventricular septum (approximately 7-cm resection).6,12 In some patients, surgical myectomy is combined with additional mitral apparatus reparative procedures, but mitral valve replacement is fortunately rarely required.12,13 The operation results in almost complete abolition of the gradient and mitral regurgitation, and at 5 years approximately 95% of patients are New York Heart Association functional class I or II. Late survival is excellent and equal to age- and sex-matched controls and at 10 years freedom from HOCM-related death is 95% and freedom from sudden cardiac death is 99%.14,15 In a nonrandomized comparison, survival in a Mayo Clinic cohort undergoing surgical myectomy was significantly better than among a nonoperated cohort from Minneapolis, Florence, and Naples with HOCM. It appeared that surgery eliminated the excess mortality due to obstruction per se. Nonetheless, although the late results of septal myectomy suggest that mortality from HOCM-related, sudden cardiac death and ICD discharge rates are lower than expected; there are no randomized trial data to support mortality as the sole indication for surgery. In the modern era, the indications for surgery are unchanged; namely, the presence of symptoms resulting in a significant impairment of quality of life despite an adequate trial of medical therapy. The recent American College of Cardiology Foundation/American Heart Association guidelines gave surgical myectomy a IIA recommendation and stated that this “is the first consideration for the majority of eligible patients with HOCM”.6
ALCOHOL SEPTAL ABLATIONASA is a much newer procedure first reported in 1995.16 The indications are similar to those for myectomy; namely, the presence of limiting symptoms after an adequate trial of medical therapy, and the technique is particularly useful in patients who are poor surgical candidates (guideline recommendation IIA). In patients who are good surgical candidates but who have a strong preference to avoid surgery after a “thorough and balanced discussion”, ASA received a grade IIB indication.6
A recent Mayo Clinic study demonstrated the considerable learning curve influencing the results of ASA,2 and the complications rate when compared with an age- and sex-matched group undergoing septal myectomy is higher, especially the need for permanent pacemaker implantation and a lower but significant rate of tamponade and early ventricular arrhythmias.17 Gradient reduction and improvements in symptoms are well documented, but the reduction in gradient and the degree of symptomatic relief still appears to be somewhat better after myectomy, especially in younger patients < 65 years.
There is still a lingering concern over the risk of malignant ventricular arrhythmias after ASA. This concern is based on the morphology of the infarcted area, as visualized by magnetic resonance imaging18 and other studies, which imply that the rate of ICD discharges is substantially higher after ASA.6,14,19 It is reassuring that, in this 10-year follow-up study by De la Torre Hernández et al, the rate of sudden cardiac death or ICD discharge is low, as it is in a recent 7-year follow-up study by the Mayo Clinic.20
DUAL-CHAMBER PERMANENT PACINGImplantation of a dual-chamber pacemaker was proposed as an alternative treatment for patients with symptomatic HOCM,6 but much of the perceived improvement was subsequently shown to be primarily a placebo effect.21 Currently, permanent pacemaker implantation is only indicated for patients who have conduction disease or who are not candidates for septal reduction therapy. Nonetheless, in symptomatic patients with an ICD in place for other reasons, a trial of dual-chamber pacing is reasonable.6
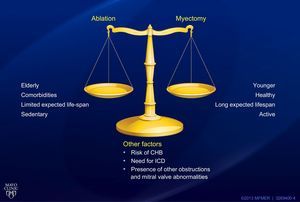
The guidelines repeatedly stress the need for HOCM to be managed in centers of excellence with access to all diagnostic and therapeutic modalities, including genetic counseling. Pending further data, our own approach to septal reduction therapy is to maintain surgical septal myectomy as the gold standard in younger, healthy patients with a long expected lifespan and who are active (Figure 2). ASA is an excellent alternative in older and sedentary patients and those who, on the basis of age and comorbidities, are suboptimal surgical candidates. Other factors, such as preprocedural risk of complete heart block and the presence of intrinsic abnormalities of the mitral valve apparatus and the need for an ICD, would also influence the decision.
CONCLUSIONSIn regard to the management of symptomatic patients with HOCM, what are the next steps to be taken? There is certainly a need for newer pharmacologic approaches, but I suspect that we have a long wait ahead of us before these enter the clinical arena. In regard to the comparison of septal myectomy vs alcohol ASA, a randomized controlled trial would certainly be the “gold standard”, but such a trial is unlikely to ever be undertaken.22 We already know the short-term results of both procedures, and randomization of a large number of patients with a lengthy period of follow-up is simply not realistic. There is clearly a need for observational registries4 that include detailed information on the technical aspects of both procedures and adverse outcomes. One hopes that this Spanish multicenter experience can be expanded into a much larger national registry including all HOCM patients treated pharmacologically and with both forms of septal reduction therapy. This could be an important contributor to an understanding of comparative efficacy.
In 2014, we now know much more about what we do not know. Available comparisons of ASA and surgical septal myectomy are retrospective and limited and, as such, are susceptible to publication and selection bias.4 The time has come for large, prospective, and collaborative studies that can provide the requisite data for assessments of comparative efficacy. For us to practice evidence-based medicine, the basic foundation must be a strong database.
CONFLICTS OF INTERESTNone declared