COVID-19 is currently causing high mortality and morbidity worldwide. Information on cardiac injury is scarce. We aimed to evaluate cardiovascular damage in patients with COVID-19 and determine the correlation of high-sensitivity cardiac-specific troponin T (hs-cTnT) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) with the severity of COVID-19.
MethodsWe included 872 consecutive patients with confirmed COVID-19 from February to April 2020. We tested 651 patients for high-sensitivity troponin T (hs-TnT) and 506 for NT-proBNP on admission. Cardiac injury was defined as hs-TnT> 14ng/L, the upper 99th percentile. Levels of NT-proBNP> 300 pg/mL were considered related to some extent of cardiac injury. The primary composite endpoint was 30-day mortality or mechanical ventilation (MV).
ResultsCardiac injury by hs-TnT was observed in 34.6% of our COVID-19 patients. Mortality or MV were higher in cardiac injury than noncardiac injury patients (39.1% vs 9.1%). Hs-TnT and NT-proBNP levels were independent predictors of death or MV (HR, 2.18; 95%CI, 1.23-3.83 and 1.87 (95%CI, 1.05-3.36), respectively) and of mortality alone (HR, 2.91; 95%CI, 1.211-7.04 and 5.47; 95%CI, 2.10-14.26, respectively). NT-ProBNP significantly improved the troponin model discrimination of mortality or MV (C-index 0.83 to 0.84), and of mortality alone (C-index 0.85 to 0.87).
ConclusionsMyocardial injury measured at admission was a common finding in patients with COVID-19. It reliably predicted the occurrence of mortality and need of MV, the most severe complications of the disease. NT-proBNP improved the prognostic accuracy of hs-TnT.
Keywords
In December 2019, the first cases of pneumonia of unknown origin were noted in Wuhan, China. A novel coronavirus—called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) after its similarity to the previous SARS virus—caused what we now know as COVID-19 disease.1 Spain is one of the countries with the highest number of infections, along with the highest number of reported deaths per million inhabitants.2
Coronaviruses are known to affect the cardiovascular system3 and early publications are currently showing that the rates of complications and mortality of COVID-19 are higher in patients with pre-existing cardiovascular risk factors or cardiovascular disease.4,5 In addition, 2 studies have shown that myocardial injury and cardiovascular risk factors were associated with a worse prognosis in patients with COVID-19 in 2 Chinese cohorts.6,7 It has been hypothesized that the virus can cause cardiac injury,8 but data on this is scarce and clinical and prognostic consequences remain unclear.
Troponin has been used to determine the extent of cardiac injury, but determination of the level of N-terminal pro-B-type natriuretic peptide (NT-proBNP) on admission of COVID-19 patients could also help to better stratify the risk of in-hospital mortality or mechanical ventilation (MV).
Our objective was to determine the accuracy of the prediction of short-term mortality or MV by combining the cardiac injury information of high-sensitivity cardiac-specific troponin-T (hs-cTnT) with NT-pro-BNP measured on admission in COVID-19 patients.
MethodsStudy design and data sourcesWe designed a cohort study of consecutive COVID-19 patients diagnosed by reverse-transcription polymerase chain reaction at Hospital del Mar, Barcelona, Spain, from February 27 to April 7, 2020. Patients were followed up for clinical outcomes until May 1, 2020.
Demographic characteristics (age and sex), comorbidities, laboratory determinations (including cardiac biomarkers), chest radiography, electrocardiographic findings, treatments, complications and outcomes were collected into an electronic data capture system.
Laboratory testsA confirmed case of COVID-19 was defined by a positive result on a reverse-transcription polymerase chain reaction assay of a specimen collected from a nasopharyngeal swab. Only laboratory-confirmed cases were included. Reverse-transcription polymerase chain reaction assays were performed in accordance with World Health Organization interim guidance.9
Laboratory tests included blood count, coagulation tests, liver and renal function analyses, electrolytes, C-reactive protein, procalcitonin, lactate dehydrogenase, creatine kinase, D-dimer and the 2 tested cardiac markers, hs-cTnT and NT-proBNP, which were measured within 48hours of admission. An electrochemiluminescence-based immunoanalytical system, Elecsys 2010 (Roche Diagnostics Ltd, Mannheim, Germany), was used to determine plasma levels of NT-proBNP and hs-cTnT.
Cardiac marker definitions and study outcomesAccording to the Fourth Universal Definition of Myocardial Infarction (2018), cardiac injury was diagnosed if serum levels of cardiac biomarkers (eg, hs-cTnT) were above the 99th percentile upper reference limit, (> 14.0 ng/L), as recommended by the manufacturer10,11 and regardless of new abnormalities on electrocardiography and echocardiography. For NT-proBNP, positivity was considered if serum levels were above the limit for ruling out heart failure in the acute setting, which is <300 pg/mL.12 Thus, the cutoff was set at ≥ 300 pg/mL.
Acute respiratory distress syndrome was diagnosed according to the Berlin criteria as acute-onset hypoxemia (ratio of arterial oxygen partial pressure to fractional inspired oxygen expressed as a fraction <300) associated with bilateral pulmonary opacities on chest imaging that were not fully explained by congestive heart failure or other forms of volume overload.13
The primary endpoint was the composite of death or the need for MV at 30 days after COVID-19 diagnosis, as also used in previous studies to assess the severity of COVID-19 infectious disease.5 In addition, we analyzed the capacity of the biomarkers to predict mortality alone.
Statistical analysisCategorical variables are summarized as counts and percentages, and continuous variables as the number of nonmissing observations, the mean and standard deviation (SD), or the median and interquartile range [IQR], depending on the distribution of the variable. Normality of distributions was tested by normal Q-Q plots. Patient characteristics were compared between hs-cTnT (cutoff point> 14 ng/L), NT-proBNP (cutoff point> 300 pg/L) and outcome status categories (composite endpoint including death or MV) by the Student t test or Mann-Whitney U test for continuous variables, and by the Pearson chi-squared test for categorical variables.
Kaplan-Meier survival curves for death or the composite endpoint were plotted, and the log-rank test was computed to assess differences between groups of hs-cTNT and NT-pro-BNP.
The adjusted hazard ratio of death and the composite endpoint for hs-cTnT and NT-proBNP status was analyzed using Cox proportional hazard models. The models were adjusted for potential confounders selected by stepwise backward elimination, among patient characteristics that were significantly (P <.10) associated with an hs-cTnT- or NT-proBNP-positive status as well as with the composite endpoint. Age was excluded from the list of potential confounders because it was among the criteria used to give patients access to an intensive care unit and MV, which is part of the composite endpoint outcomes.
The assumption of proportionality of hazards from the Cox models was checked. The hazard ratio for laboratory determinations was calculated by 10 or 100 measurement units change. The diagnostic test accuracy for hs-cTnT and NT-proBNP and mortality and death or mechanical ventilation is illustrated in . Kaplan-Meier and Cox models took into account the delay between symptom onset and admission by the left-truncation approach. The c-statistic was calculated to analyze the discriminatory ability of the adjusted models. Hosmer-Lemeshow test taking into account right-censoring was computed to assess model calibration. Continuous, categorical (into 3 risk groups defined by tertiles) and clinical net reclassification indexes were computed to assess whether the inclusion of NT-proBNP in a model with hs-cTnT and confounders improved the classification of individual outcomes. P values <.05 were considered statistically significant. All tests were performed with R (3.5.3) (R: A language and environment for statistical computing; R Foundation for Statistical Computing, Vienna, Austria).14
This study was performed in accordance with the provisions of the Declaration of Helsinki, ISO 14155, and clinical practice guidelines. The study protocol was approved by the Institutional Ethics Committee and the research commission of our hospital. The need for written informed consent was waived in light of the urgent need to collect data and the infectious disease hazard.
ResultsThe flowchart in figure 1 shows the patient recruitment process. Less than 6% of the 923 diagnosed COVID-19 patients had to be excluded from this study. Of the remaining 872 cases, 75% could be tested on admission for hs-cTnT and 58% for NT-proBNP. Among these patients, 34.6% and 36.2%, respectively, showed elevated levels of these biomarkers. Mortality or MV was higher in patients with hs-cTnT> 14 ng/L than in the remaining patients (39.1% vs 9.1%), as well as in patients with NT-proBNP> 300 pg/L than in the remaining patients (42.6% vs 6.8%) ().
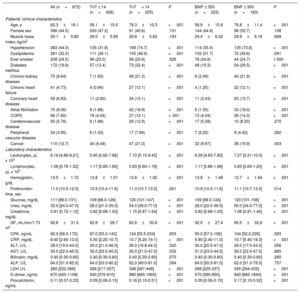
Baseline and demographic characteristics and laboratory findingsThe baseline characteristics of the population are summarized in table 1 by admission levels of hs-TnT and NT-proBNP. Patients with cardiac injury as defined by hs-TnT levels, were significantly older, with higher prevalence rates of cardiovascular risk factors and a previous history of cardiovascular disease. Patients with cardiac injury as defined by baseline NT-proBNP levels had a similar clinical profile to the corresponding hs-cTnT-defined population.
Clinical and laboratory characteristics at admission in all patients and by admission hs-cTnT> 14 ng/L and by NT-proBNP> 300 pg/L
| All (n=872) | TnT ≤ 14 (n=426) | TnT> 14 (n=225) | P | BNP ≤ 300 (n=323) | BNP ≤ 300 (n=183) | P | |
|---|---|---|---|---|---|---|---|
| Patients’ clinical characteristics | |||||||
| Age, y | 62.3±18.1 | 56.1±15.0 | 78.3±10.3 | <.001 | 56.9±15.8 | 76.8±11.4 | <.001 |
| Female sex | 386 (44.5) | 200 (47.2) | 91 (40.6) | .131 | 144 (44.9) | 96 (52.7) | .108 |
| Muscle mass index, kg/m2 | 29.1±5.80 | 29.0±5.89 | 29.8±5.62 | .193 | 29.4±6.02 | 29.8±6.16 | .669 |
| Hypertension | 383 (44.0) | 135 (31.8) | 168 (74.7) | <.001 | 114 (35.4) | 135 (73.8) | <.001 |
| Dyslipidemia | 281 (32.3) | 111 (26.1) | 105 (46.9) | <.001 | 102 (31.7) | 72 (39.6) | .091 |
| Ever smoker | 208 (24.5) | 96 (23.0) | 56 (25.6) | .526 | 78 (24.6) | 44 (24.7) | 1.000 |
| Diabetes mellitus | 172 (19.8) | 57 (13.4) | 73 (32.4) | <.001 | 49 (15.3) | 54 (29.5) | <.001 |
| Chronic kidney disease | 75 (8.64) | 7 (1.65) | 48 (21.3) | <.001 | 8 (2.49) | 40 (21.9) | <.001 |
| Chronic heart failure | 41 (4.73) | 4 (0.94) | 27 (12.1) | <.001 | 4 (1.25) | 22 (12.1) | <.001 |
| Coronary heart disease | 59 (6.83) | 11 (2.60) | 34 (15.1) | <.001 | 11 (3.43) | 25 (13.7) | <.001 |
| Atrial fibrillation | 70 (8.06) | 8 (1.88) | 42 (18.8) | <.001 | 5 (1.55) | 33 (18.0) | <.001 |
| COPD | 66 (7.60) | 18 (4.24) | 27 (12.1) | < .001 | 13 (4.04) | 26 (14.3) | <.001 |
| Cerebrovascular disease | 50 (5.76) | 8 (1.88) | 29 (12.9) | <.001 | 17 (5.28) | 15 (8.20) | .270 |
| Peripheral vascular disease | 34 (3.95) | 6 (1.43) | 17 (7.66) | <.001 | 7 (2.20) | 8 (4.42) | .262 |
| Cancer | 110 (12.7) | 40 (9.48) | 47 (21.0) | <.001 | 32 (9.97) | 36 (19.9) | .003 |
| Laboratory characteristics | |||||||
| Leukocytes, μL x 103 | 6.16 [4.86-8.21] | 5.90 [4.82-7.86] | 7.10 [5.15-9.45] | <.001 | 6.28 [4.83-7.83] | 7.27 [5.21-10.0] | <.001 |
| Lymphocytes, μL x 103 | 1.08 [0.76-1.52] | 1.17 [0.85-1.60] | 0.83 [0.60-1.19] | <.001 | 1.17 [0.86-1.68] | 0.83 [0.60-1.20] | <.001 |
| Hemoglobin, g/dL | 13.5±1.72 | 13.8±1.51 | 12.8±1.92 | <.001 | 13.8±1.48 | 12.7±1.94 | <.001 |
| Prothrombin time, sec | 11.0 [10.5-12.0] | 10.9 [10.4-11.6] | 11.3 [10.7-13.0] | .001 | 10.9 [10.5-11.6] | 11.1 [10.7-13.0] | .014 |
| Glucose, mg/dL | 111 [98.0-131] | 109 [98.0-126] | 120 [101-147] | <.001 | 109 [98.0-124] | 120 [101-156] | <.001 |
| Urea, mg/dL | 33.0 [24.0-47.0] | 28.0 [21.0-35.0] | 54.0 [39.0-77.0] | <.001 | 28.0 [22.0-36.5] | 50.0 [34.0-77.0] | <.001 |
| Creatinine, mg/dL | 0.91 [0.72-1.12] | 0.82 [0.68-1.00] | 1.15 [0.87-1.54] | <.001 | 0.82 [0.68-1.03] | 1.06 [0.81-1.40] | <.001 |
| GF, mL/min/1.73 m2 | 82.6±31.0 | 92.8±26.7 | 62.6±30.8 | <.001 | 92.5±27.4 | 65.5±32.8 | <.001 |
| CPK, ng/mL | 92.0 [56.0-170] | 87.0 [55.0-143] | 124 [55.5-234] | .003 | 95.0 [57.0-156] | 104 [52.2-226] | .593 |
| CRP, mg/dL | 6.40 [2.60-13.0] | 5.50 [2.20-10.7] | 10.7 [5.20-19.1] | <.001 | 5.90 [2.40-11.0] | 10.7 [5.40-19.3] | <.001 |
| ALT, U/L | 28.0 [19.0-44.0] | 30.0 [21.0-46.0] | 26.0 [16.8-44.2] | .020 | 30.0 [20.5-47.0] | 26.0 [17.0-43.0] | .056 |
| AST, U/L | 30.0 [22.0-45.5] | 32.0 [23.0-45.5] | 30.0 [21.0-47.0] | .532 | 31.0 [23.0-44.5] | 30.0 [22.0-47.5] | .885 |
| Bilirubin, mg/dL | 0.40 [0.30-0.60] | 0.40 [0.30-0.60] | 0.40 [0.30-0.60] | .075 | 0.40 [0.30-0.60] | 0.40 [0.30-0.60] | .260 |
| ALP, U/L | 64.0 [51.0-82.0] | 64.0 [53.0-80.2] | 62.0 [49.0-81.0] | .394 | 64.0 [50.5-81.5] | 62.0 [51.0-79.5] | .757 |
| LDH U/L | 280 [222-366] | 268 [217-337] | 348 [267-446] | <.001 | 268 [225-337] | 345 [264-432] | <.001 |
| D-dimer, ng/mL | 670 [420-1108] | 540 [370-910] | 980 [665-1905] | <.001 | 570 [390-950] | 940 [660-1840] | <.001 |
| Procalcitonin, ng/mL | 0.11 [0.07-0.23] | 0.09 [0.06-0.15] | 0.18 [0.10-0.51] | <.001 | 0.09 [0.06-0.15] | 0.17 [0.10-0.52] | <.001 |
ALT, alanine aminotransferase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; CRP, C-reactive protein; COPD, Chronic Obstructive Pulmonary Disease; CPK, creatine phosphokinase; GF, glomerular filtrate; hs-cTnT, high-sensitivity cardiac-specific troponin T; LDH, lactate dehydrogenase; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Values are expressed as No. (%), or median [interquartile range].
Laboratory findings are summarized in table 1 by admission levels of hs-TnT and NT-proBNP. Patients with positive hs-cTnT and NT-proBNP levels showed more severe inflammatory responses.
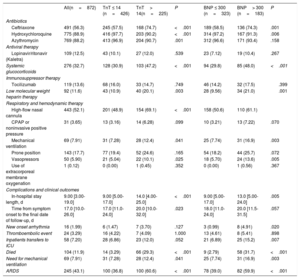
Management and outcomesManagement details and hospital outcomes are summarized in table 2 by admission levels of hs-TnT and NT-proBNP. Almost two thirds (62%) of patients required some degree of oxygen therapy, with 55 patients (7%) requiring MV. Patients with cardiac injury received more hydroxychloroquine, azithromycin, systemic glucocorticoids, and low-molecular-weight heparin. In addition, they required more support from either a high-flow nasal cannula or invasive mechanical ventilation, as well as vasopressors. Median length of hospital stay was 9 [IQR 3–19] days. One hundred and four patients (12%) died during admission (figure 1).
Patient treatment, complications and outcome characteristics by admission hs-cTnT> 14 ng/L and by NT-proBNP> 300 pg/L
| All(n=872) | TnT ≤ 14 (n=426) | TnT> 14(n=225) | P | BNP ≤ 300 (n=323) | BNP> 300 (n=183) | P | |
|---|---|---|---|---|---|---|---|
| Antibiotics | |||||||
| Ceftriaxone | 491 (56.3) | 245 (57.5) | 168 (74.7) | <.001 | 189 (58.5) | 136 (74.3) | .001 |
| Hydroxychloroquine | 775 (88.9) | 416 (97.7) | 203 (90.2) | <.001 | 314 (97.2) | 167 (91.3) | .006 |
| Azythromycin | 769 (88.2) | 413 (96.9) | 204 (90.7) | .001 | 312 (96.6) | 171 (93.4) | .158 |
| Antiviral therapy | |||||||
| Lopinavir/ritonavir (Kaletra) | 109 (12.5) | 43 (10.1) | 27 (12.0) | .539 | 23 (7.12) | 19 (10.4) | .267 |
| Systemic glucocorticoids | 276 (32.7) | 128 (30.9) | 103 (47.2) | <.001 | 94 (29.8) | 85 (48.0) | <.001 |
| Immunosuppressor therapy | |||||||
| Tocilizumab | 119 (13.6) | 68 (16.0) | 33 (14.7) | .749 | 46 (14.2) | 32 (17.5) | .399 |
| Low molecular weight heparin therapy | 92 (11.6) | 43 (10.9) | 40 (20.1) | .003 | 28 (9.56) | 34 (21.0) | .001 |
| Respiratory and hemodynamic therapy | |||||||
| High-flow nasal cannula | 443 (52.1) | 201 (48.9) | 154 (69.1) | <.001 | 158 (50.6) | 110 (61.1) | |
| CPAP or noninvasive positive pressure | 31 (3.65) | 13 (3.16) | 14 (6.28) | .099 | 10 (3.21) | 13 (7.22) | .070 |
| Mechanical ventilation | 69 (7.91) | 31 (7.28) | 28 (12.4) | .041 | 25 (7.74) | 31 (16.9) | .003 |
| Prone position | 143 (17.7) | 77 (19.4) | 52 (24.6) | .165 | 54 (18.2) | 44 (25.7) | .072 |
| Vasopressors | 50 (5.90) | 21 (5.04) | 22 (10.1) | .025 | 18 (5.70) | 24 (13.6) | .005 |
| Use of extracorporeal membrane oxygenation | 1 (0.12) | 0 (0.00) | 1 (0.45) | .352 | 0 (0.00) | 1 (0.56) | .367 |
| Complications and clinical outcomes | |||||||
| In-hospital stay length, d | 9.00 [3.00-19.0] | 9.00 [5.00-17.0] | 14.0 [4.00-25.0] | <.001 | 9.00 [5.00-17.0] | 13.0 [5.00-24.0] | .005 |
| Time from symptom onset to the final date of follow-up, d | 17.0 [10.0-26.0] | 17.0 [11.0-24.0] | 20.0 [10.0-32.0] | .023 | 18.0 [11.0-24.0] | 20.0 [11.5-31.5] | .057 |
| New onset arrhythmia | 16 (1.99) | 6 (1.47) | 7 (3.70) | .127 | 3 (0.99) | 8 (4.91) | .020 |
| Thromboembolic event | 24 (3.29) | 16 (4.22) | 7 (4.09) | 1.000 | 13 (4.61) | 8 (5.41) | .898 |
| Inpatients transfers to ICU | 58 (7.20) | 28 (6.86) | 23 (12.0) | .052 | 21 (6.89) | 25 (15.2) | .007 |
| Died | 104 (11.9) | 14 (3.29) | 66 (29.3) | <.001 | 9 (2.79) | 58 (31.7) | <.001 |
| Need for mechanical ventilation | 69 (7.91) | 31 (7.28) | 28 (12.4) | .041 | 25 (7.74) | 31 (16.9) | .003 |
| ARDS | 245 (43.1) | 100 (36.8) | 100 (60.6) | <.001 | 78 (39.0) | 82 (59.9) | <.001 |
ARDS, acute respiratory distress syndrome; CPAP, continuous positive airway pressure; hs-cTnT, high-sensitivity cardiac-specific troponin T; ICU, intensive care unit; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Values are expressed as No. (%), BNP ≤ 300 or median [interquartile range].
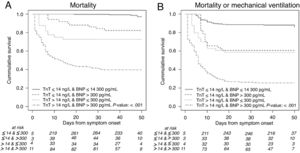
Patients with cardiac injury had longer hospital stays. The rates of mortality and of the composite endpoint including mortality or the need for MV were significantly higher among patients with vs without cardiac injury, as shown in table 2 and the Kaplan-Meier survival curves in figure 2.
Kaplan-Meier 50-day survival curves for mortality during the time from symptom onset by 4 combinations of high-sensitivity troponin T (hs-TnT) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels (A), and for the composite of mortality or mechanical ventilation by 4 combinations of hs-cTnT and NT-proBNP levels (B).
As illustrated in , hs-cTnT levels were significantly associated with COVID-19 severity. Patients with mild symptoms and those who were discharged to the hospital-at-home program had no cardiac injury. One-quarter of patients needing hospitalization but no respiratory support showed cardiac injury. Patients with respiratory support but no mechanical ventilation constituted one-third of cases and nearly half of patients requiring MV had cardiac injury. More than 80% of patients who died had positive levels of hs-cTnT at admission. We found similar results for the NT-proBNP values.
NT-proBNP showed a fair correlation with hs-cTnT when considered as continuous data (Spearman's R=0.64, P-value <.001), as shown in .
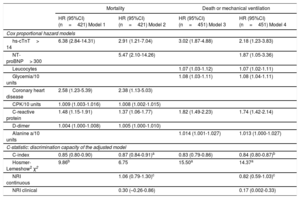
The Cox proportional hazard regression models (table 3) showed that serum levels of hs-cTnT> 14 ng/L and NT-proBNP> 300 pg/mL were significant independent predictors of mortality and of the composite of mortality or need for MV. Other factors contributed independently to mortality, namely, history of coronary heart disease, creatine phosphokinase levels, C-reactive protein and D-dimer. Glycemia and C-reactive protein were significantly associated with the composite endpoint, in addition to hs-TnT and NT-proBNP.
Cox proportional hazard models
| Mortality | Death or mechanical ventilation | |||
|---|---|---|---|---|
| HR (95%CI)(n=421) Model 1 | HR (95%CI)(n=421) Model 2 | HR (95%CI)(n=451) Model 3 | HR (95%CI)(n=451) Model 4 | |
| Cox proportional hazard models | ||||
| hs-cTnT> 14 | 6.38 (2.84-14.31) | 2.91 (1.21-7.04) | 3.02 (1.87-4.88) | 2.18 (1.23-3.83) |
| NT-proBNP> 300 | 5.47 (2.10-14.26) | 1.87 (1.05-3.36) | ||
| Leucocytes | 1.07 (1.03-1.12) | 1.07 (1.02-1.11) | ||
| Glycemia/10 units | 1.08 (1.03-1.11) | 1.08 (1.04-1.11) | ||
| Coronary heart disease | 2.58 (1.23-5.39) | 2.38 (1.13-5.03) | ||
| CPK/10 units | 1.009 (1.003-1.016) | 1.008 (1.002-1.015) | ||
| C-reactive protein | 1.48 (1.15-1.91) | 1.37 (1.06-1.77) | 1.82 (1.49-2.23) | 1.74 (1.42-2.14) |
| D-dimer | 1.004 (1.000-1.008) | 1.005 (1.000-1.010) | ||
| Alanine a/10 units | 1.014 (1.001-1.027) | 1.013 (1.000-1.027) | ||
| C-statistic: discrimination capacity of the adjusted model | ||||
| C-index | 0.85 (0.80-0.90) | 0.87 (0.84-0.91)a | 0.83 (0.79-0.86) | 0.84 (0.80-0.87)b |
| Hosmer-Lemeshow2 χ2 | 9.86b | 6.75 | 15.50a | 14.37a |
| NRI continuous | 1.06 (0.79-1.30)c | 0.82 (0.59-1.03)c | ||
| NRI clinical | 0.30 (–0.26-0.86) | 0.17 (0.002-0.33) | ||
Adjusted hazard ratio of mortality for hs-cTnT> 14 (Model 1) and adding NT-proBNP> 300 (Model 2) by multivariate cox regression analysis in patients with COVID-19. Adjusted hazard ratio of composite endpoint death or mechanical ventilation for hs-cTnT> 14 (Model 3) and adding NT-proBNP> 300 (Model 4) by multivariate cox regression analysis in patients with COVID-19. All measurements were taken on admission. C-statistic: Calculated to analyze the discriminatory ability of the adjusted model.
95%CI, 95% confidence interval; CPK, creatine phosphokinase; HR, hazard ratio; hs-cTnT, high-sensitivity cardiac-specific troponin T; NRI, net reclassification indexes; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
In the Cox proportional hazard regression models presented in table 3, the inclusion of NT-proBNP (cutoff value, 300 pg/mL) significantly improved the discrimination (C-index) and continuous net reclassification indexes for both mortality alone and the composite endpoint (table 3).
DiscussionSome interesting aspects of this large prospective registry should be emphasized. First, this is the largest COVID-19 population tested for cardiac markers to analyze myocardial injury. Second, we observed a high prevalence (34.6%) of cardiac injury by hs-cTnT in patients with COVID-19. Third, we confirmed a strong and independent association of hs-cTnT> 14 ng/L with the severity of COVID-19. Fourth, we show for the first time that NT-proBNP improved the prognostic accuracy of hs-cTnT for the outcomes analyzed. These findings suggest that the measurement of biomarkers of cardiac damage on admission for SARS-CoV-2 infection may help in risk stratification by identifying a subset of patients with cardiac injury with a high risk of poor COVID-19 prognosis.
Initial data from hospitalized patients with COVID-19 pneumonia in Wuhan, China, showed that 12% (5 out of 41) of patients developed acute cardiac injury, and that patients admitted to an intensive care unit were more likely to develop cardiac injury (31%) than nonintensive care unit patients (4%).1 More recently, Shi et al.6,15 observed an almost 20% prevalence of cardiac injury in 416 patients hospitalized for COVID-19, defined as blood levels of hs-cTnT above the 99th percentile. Our study of 651 patients suggests that the proportion of patients with cardiac injury is higher (35%) than this observation by Shi et al., using the same hs-cTnT level threshold. Furthermore, in our registry, we tested a second cardiac marker, NT-proBNP levels above 300 pg/mL. The use of this threshold gave a similar percentage (36%) of patients with cardiac injury as the analysis with hs-cTnT. Previous outbreaks of viral infections such as SARS, MERS (Middle East Respiratory Syndrome) and influenza virus were found to be more severe and have higher mortality in patients with a history of cardiovascular disease. However, the exact contributions of these viruses to cardiac injury are unclear, and there are only a few reports of myocarditis in the literature.16–21
Consistent with previously published data, patients with cardiac injury were older, with more cardiovascular risk factors and a higher prevalence of cardiovascular disease. Their blood tests showed higher levels of inflammatory parameters, such as leukocytes and C-reactive protein. They also had a higher frequency of chronic kidney disease, and previous cancer and chronic obstructive pulmonary disease (COPD).1–5 In agreement with findings from other series, patients with myocardial injury were more likely to require hospital stay, frequently in an intensive care unit,1 with more severe infection. Nearly half of patients requiring mechanical ventilation had cardiac injury and more than 80% of patients who died had positive levels at admission. In contrast, patients with mild symptoms had no cardiac injury, and therefore we concluded that cardiac markers increased according to the severity of the infection.
Myocardial injury can be seen in critically ill patients in other circumstances,22–24 including systemic infection. It is argued that the rise in cardiac markers in patients with COVID-19 could be due to multiple factors.8 Few patients develop fulminant myocarditis as a result of direct myocardial infection by the virus.25–27 However, most patients appear to be affected by inflammation and oxidative stress through a cytokine storm that causes coagulopathy and microangiopathy, leading to perfusion defects and myocardial injury.28,29 Another potential pathophysiological explanation is the imbalance between high oxygen demand (due to tachycardia and fever) and low oxygen supply (due to hypoxemia and respiratory failure) that occurs during the infection, which could lead to a type 2 myocardial infarction.11,28,30 This could explain why cardiac damage was observed in patients with a more aggressive infection, probably among those who were older and had previously had cardiac disease or exhibited cardiovascular risk factors and more comorbidities.
As reported in other studies, hs-cTnT levels were a strong predictor of in-hospital death.30 In addition, our registry is the first to show that hs-cTnT and NT-proBNP levels at admission are independent and complementary predictors of mortality or the need for mechanical ventilation. We found that NT-proBNP showed a fair correlation with hs-cTnT (Spearman's R=0.64). Furthermore, we observed that NT-proBNP improved the prognostic accuracy of hs-cTnT for the outcomes analyzed. It has been reported that elevation of NT-proBNP is not necessarily disease-specific, rather reflecting hemodynamic deterioration, myocardial wall stress, myocardial ischemia, derangements in volume loading conditions, and renal function.31 Thus, NT-proBNP elevation might reflect more extensive cardiovascular injury in COVID-19 disease.
Whichever the etiology, the presence of cardiac injury, as measured by hs-cTnT and NT-proBNP, early in admission is a predictor of severe complications in COVID-19 infection and should prompt increased vigilance to anticipate the need for advanced treatments. Deng et al.32 observed a rise in cardiac biomarkers preceding the death of severely ill COVID-19 patients. However, they analyzed troponin at any time during the admission of patients, but not specifically the levels of cardiac biomarkers at admission. All of the few published works had a high percentage of patients still hospitalized at the time of publication, whereas in our case only 2% of the patients were not yet discharged, ensuring the completeness of the follow-up. In addition, the present study included patients with disease severity ranging from mild to critical.
LimitationsOur study has some limitations. First, asymptomatic patients were not included in this registry, which confers a selection bias. However, we focused on patients who were diagnosed in hospital, some of whom were discharged to home for care there. Second, because this is an observational study, no causal inference for the association between cardiac injury and severity of COVID-19 infection can be drawn. Clinical trials to demonstrate whether this association is useful in guiding treatments are needed to further comprehend the significance of our findings. Third, this is a single-centre study with a limited number of patients relative to the magnitude of this pandemic. Larger studies in upcoming months should add to our results.
ConclusionsMyocardial injury is a common finding in patients admitted for COVID-19. It predicts the development of more severe disease, including the need for invasive mechanical ventilation and the risk of in-hospital death. NT-proBNP substantially improves the prognostic accuracy of hs-cTnT. It would be worth measuring hs-cTnT and NT-proBNP as markers of cardiovascular injury early after admission to stratify risk and to anticipate the need for advanced therapies.
CONFLICTS OF INTERESTThe authors declare no conflict of interest.
- –
Coronaviruses are known to affect the cardiovascular system and early publications have shown that the rates of complications and mortality of COVID-19 are higher in patients with pre-existing cardiovascular risk factors or cardiovascular disease.
- –
Two previous studies have shown that myocardial injury and cardiovascular risk factors were associated with a worse prognosis in patients with COVID-19 in 2 Chinese cohorts.
- –
It has been hypothesized that the virus can cause cardiac injury, but data on this issue are scarce and clinical and prognostic consequences remain unclear.
- –
This is the largest COVID-19 population tested for cardiac markers to analyze myocardial injury.
- –
This study is the first to demonstrate, in Europe, a relatively high prevalence of cardiac injury by hs-cTnT in patients with COVID-19.
- –
This study confirmed a strong and independent association of hs-cTnT> 14 ng/L with the severity of COVID-19.
- –
This study showed for the first time that NT-proBNP improved the prognostic accuracy of hs-cTnT for death and need for mechanical ventilation.
We thank Dr Julio Pascual and Dr Isabel Cirera from Hospital del Mar for their invaluable help in identifying the patients with a positive test result for SARS-CoV-2.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2020.09.011