Bleeding complications are associated with worse prognosis in patients with acute coronary syndrome (ACS).1 In recent years, different scales have been developed to predict in-hospital bleeding complications in ACS,2–4 and these scales have been shown to have acceptable predictive power in different scenarios.5 However, these scales have not been validated for predicting bleeding complications outside the hospital setting.
The objective of this study was to assess prediction of bleeding by the CRUSADE,2 Mehran,3 and ACTION4 bleeding risk scales 1 year after hospital discharge and to compare the predictive power with that shown by the 3 scales for predicting in-hospital bleeding events.
This was a retrospective study of prospectively followed patients admitted to the coronary unit of a tertiary hospital for ACS between October 2009 and April 2014. The scores on the CRUSADE,2 Mehranm,3 and ACTION4 scales were calculated for each patient. To define in-hospital bleeding, the Bleeding Academic Research Consortium (BARC) definition was used (types 3 and 5).6
Follow-up at 1 year was undertaken through chart review or telephone contact. The development of clinically relevant bleeding events, defined as those that required hospitalization, transfusion of ≥ 1 blood pack, or suspension of antithrombotic treatment, was recorded.
The predictive power of these 3 scales for predicting in-hospital bleeding was analyzed by means of binary logistic regression analysis and by calculation of areas under curve (AUCs) of the receiver operating characteristics (ROC) curves, with comparison using the DeLong method. The predictive power of the 3 scales for bleeding during follow-up was analyzed by Fine and Gray comparison of competing risks (with death as a competing event), and by calculation of the corresponding AUC of the ROC curves, with comparison as before with the DeLong method.
Of the 1489 patients included, with a mean age of 62.5 years, 77.7% were men. Forty-nine patients (3.3%) had type 3 or 5 BARC bleeding events during hospitalization. In-hospital mortality was 6.3%. Of the 94 patients who died in hospital, 35 (37.2%) died of noncardiac causes and 5 (5.3%) died of bleeding complications.
In total, 1375 patients entered follow-up (97.9%, median follow-up time of 365 days), and during this period, 69 patients had bleeding complications and 73 died. The mean time to bleeding event after discharge was 169 days. Eight (11.6%) of these bleeding events occurred in the first 30 days, and 38 events (55.1%) were reported in the first 6 months. The most frequently reported type of bleeding after discharge was urinary bleeding in 24 patients (34.8%), followed by gastrointestinal bleeding in 16 (23.2%), respiratory system bleeding in 15 (21.7%), intracranial bleeding in 5 (7.2%), and muscle bleeding in 5 (7.2%).
Of the 73 patients who died during follow-up, 43 (58.9%) died of noncardiac causes and 4 (5.5%) due to bleeding complications.
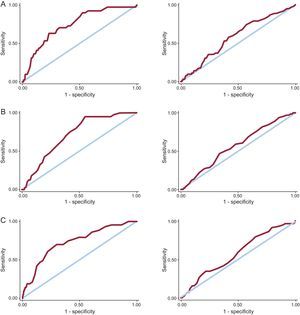
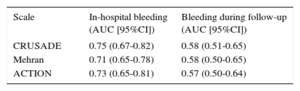
In the overall patient population, the 3 scales showed a good predictive power for in-hospital bleeding (Table), with no statistically significant differences between the different AUCs (P not significant). By contrast, the predictive power of the 3 scales for bleeding complications at 1 year after discharge in those who survived their stay in hospital was deficient, and as before there were no significant differences between the 3 AUCs (P not significant). The Figure shows the ROC curves for predicting in-hospital bleeding events during follow-up for the 3 scales.
Area Under Curve of Different Scales for Predicting Bleeding Events
| Scale | In-hospital bleeding (AUC [95%CI]) | Bleeding during follow-up (AUC [95%CI]) |
|---|---|---|
| CRUSADE | 0.75 (0.67-0.82) | 0.58 (0.51-0.65) |
| Mehran | 0.71 (0.65-0.78) | 0.58 (0.50-0.65) |
| ACTION | 0.73 (0.65-0.81) | 0.57 (0.50-0.64) |
AUC, area under ROC curve; CI, confidence interval.
Our study is subject to the limitations inherent in a single-center register, with a relatively low number of events and a homogeneous clinical management. Thus, our findings may not be applicable to populations with different characteristics and treatments. In addition, the fact that these patients are admitted to the coronary unit might imply a selection bias. The use of different bleeding definitions for the in-hospital phase and the follow-up phase after discharge is another limitation of the study. Nevertheless, in our opinion, the use of different definitions is justified by the conceptual and pathophysiological differences between in-hospital bleeding. For example, some characteristics of the definition used for bleeding after discharge (essentially, the need for hospitalization) are not applicable to a patient already in hospital. In any case, the use of different definitions cannot, we believe, explain the marked differences in AUC apparent across all 3 scales.
Recent data suggest that major bleeding after discharge for ACS is associated with mortality to the same extent as during hospitalization.1 The prediction of bleeding after discharge is extremely relevant for several other reasons, such as the availability of new and potent antiplatelet drugs, doubts as to the optimal duration of dual antiplatelet therapy after ACS, or the increasingly frequent indication of anticoagulation for atrial fibrillation or other diseases due to the progressive aging of the population. In the absence of other tools, these risk scales are the ones usually used by clinicians to select the type and duration of antithrombotic treatment after discharge. Even considering the aforementioned limitations, our data show the relatively poor performance of these scales for predicting the development of bleeding complications in the first year after ACS. In our opinion, this highlights the need for new more precise and reliable tools to stratify bleeding risk after hospitalization.
CONFLICTS OF INTERESTE. Abu-Assi is Associate Editor of Revista Española de Cardiología.