Keywords
Chronic ischemic mitral valve regurgitation (CIMR) is an increasingly important problem due to its substantial incidence and clinical repercussions.
In recent years, several studies have shown that when left uncorrected CIMR leads to a worse prognosis in patients with coronary disease. Hence, many authors currently recommend simultaneous mitral valve repair and surgical revascularization even in patients with moderate CIMR.1,2
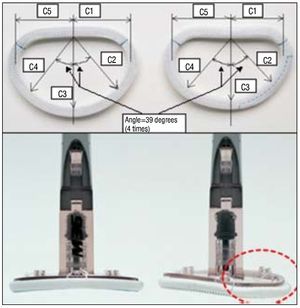
The most common mechanism in CIMR is restricted leaflet motion, especially of the posterior leaflet during systole (Carpentier's type IIIB dysfunction)3 due to subvalvular apparatus tethering.4 The Carpentier-McCarthy-Adams IMR ETlogix® prosthetic ring (CMA IMR ETlogix; Edwards Lifescience, Irvine, California, USA) was designed on the basis of observations such as these.5 This mitral ring acts directly on the asymmetric deformation that characterizes type IIIb mitral regurgitation (MR).3 Compared to conventional symmetrical rings, it increases leaflet coaptation thanks to a markedly reduced anteroposterior diameter (Figure 1).
Figure 1. Comparison of the classical Physio ring (left) with the CMA IMR ETlogix ring (right). The CMA IMR ETlogix ring is undersized, with a 14% reduction in the posteromedial axis (C1, C2 dimension). Moreover, it presents a depression at the level of the mitral P2-P3 (Figure 2B) and is smaller at the level of P2-P3 (C1, C2 dimension). Courtesy of Edwards Lifesciences.
Our objective is to analyze early and mid-term results of mitral valve repair with the CMA IMR ETlogix ring.
METHODS
Patients
Prospectively, we studied 35 consecutive patients undergoing annuloplasty in our center for type IIIb CIMR and receiving prosthetic CMA IMR ETlogix mitral rings between June 2005 and July 2008.
Baseline Echocardiography
All patients underwent preoperative transthoracic echocardiography to determine mitral valve anatomy and the underlying mitral regurgitation mechanism.
Severity of MR was evaluated prospectively in all patients on the basis of regurgitant flow area (color Doppler), regurgitant volume and effective regurgitant orifice (ERO) area. The MR grade was quantified on a 0 to 4 scale following American Society of Echocardiography criteria.6
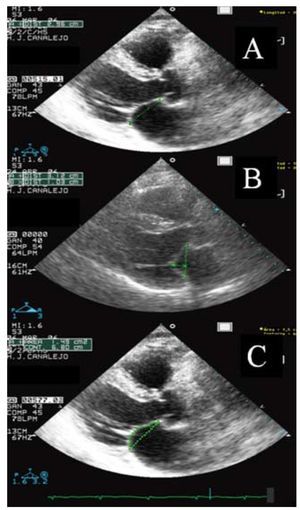
Mitral valve geometry was determined as shown in Figure 2. Mean transmitral gradient after surgical repair was calculated using continuous Doppler and mitral valvular area was estimated from pressure half-time.
Figure 2. The geometry of the mitral device is evaluated by measuring the annular diameter (A), tenting height or mitral valve closure displacement (the distance in a straight line from the point of leaflet coaptation to the theoretical height of the mitral annulus) (B), and the tethering or traction area (surface formed by the theoretical mitral annulus plane and the surface of both leaflets at closure) (C) in the long axis plane at maximum mitral valve closure in systole.
Both left ventricular ejection fraction (LVEF) and ventricular volume were determined from Simpson's biplane method.
We used intraoperative transesophageal echocardiography to determine left ventricular function and mitral valve anatomy, before and after the intervention, and to ascertain the results of mitral valve repair.
Statistical Analysis
Data are expressed as mean (SD), median [interquartile range], distribution frequency or percentage, as appropriate. Differences between preoperative and postoperative variables were analyzed with Student t test for paired data or chi-squared, as appropriate. We considered P<.05 statistically significant. We used SPSS 15.0 (SPSS Inc., Chicago, Illinois, USA) for the statistical analysis.
RESULTS
Baseline Characteristics
Mean age was 65.5 (8.7) years. Some 31.4% of patients (n=11) were in New York Heart Association (NYHA) functional class (FC) II, 48.6% (n=17) in FC III, and 20% (n=7) in FC IV. We found 91.4% of patients (n=32) were in sinus rhythm prior to surgery, whereas 8.6% (n=3) presented permanent atrial fibrillation. All patients presented significant coronary disease: 22.9% had disease of the trunk and/or three vessels (Table 1).
All patients presented grade ≥2 MR at baseline. Preoperative echocardiography showed 9 patients (25.7%) had a 0.2-0.3 cm2 ERO (grade 2 MR); 16 (45.7%) had a 0.3-0.4 cm2 ERO (grade 3 MR); and 10 (28.6%) had a >0.4 cm2 ERO (grade 4 MR). Mean preoperative regurgitant volume was 45 (18) mL, and 23 patients had ≥30 mL regurgitant volume.
Surgery
A 26 mm or 28 mm ring was deployed in 82.9% of patients. The prosthetic ring size distribution and procedures associated with mitral remodeling surgery are in Table 2. In 31 (88.6%) patients we performed myocardial revascularization with skeletonized mammary artery with a mean 1.9 (1.1) coronary artery grafts per patient.
Early Results
We recorded 1 death at ≤30 days following surgery (2.9%). The cause of death was a syndrome of systemic inflammatory response and multiorgan failure secondary to profuse bleeding and multiple transfusions. Median intensive care stay was 2 (1-21) days; median hospitalization was 7 (2-55) days.
The MR grade fell significantly in all patients (P<.001). Postoperative echocardiography at discharge confirmed absence of residual MR in 30 patients (88.8%) and grade 1 MR in 4 (10.8%). Echocardiography at discharge showed ERO had reduced to 0.008 (0.024) cm2 (P<.001) and regurgitant volume had fallen from 45 (18) to 3.3 (6.4) mL (P<.001).
Mitral geometric parameters were also significantly smaller following surgery: annular diameter (from 3.3 [0.9] to 2.1 [0.03] cm; P=.001), tenting height (from 0.8 [0.2] to 0.3 [0.1] cm; P=.001), tethering area (from 1.6 [0.4] to 0.7 [0.3] cm2; P=.001). We found no significant changes in mean LVEF (from preoperative 42.7% [16.1%] to 40.3% [10.7%] in discharge echocardiography; P=.25). However, we recorded a statistically significant reduction in mean pulmonary artery systolic pressure (PASP) from 42.9 (16) to 34.1 (10.1) mmHg in discharge echocardiography (P<.001). Mean mitral valve area fell from preoperative 4.4 (1.6) cm2 to 3 (0.8) cm2 in discharge echocardiography. Mean transmitral gradient at discharge was 5.1 (1.4) mmHg and the maximum was 12 (3.6) mmHg.
Midterm Clinical and Echocardiographic Follow-up
We achieved clinical and echocardiographic follow-up in all patients who survived surgical intervention (n=34). Median follow-up was 23 (12-44) months. No late deaths occurred during follow-up. Later check-ups revealed significant improvement in NYHA FC with 24 patients (70.6%) in FC I, 9 (26.4%) in FC II and 1 (3%) in FC III. Postoperative examinations found 29 patients (85.3%) in sinus rhythm, 4 (11.7%) in atrial fibrillation, and 1 (3%) with pacemaker rhythm.
Echocardiography revealed absence of MR in 26 (76.4%) patients but detected grade 1 MR in 6 (17.1%), grade 2 MR in 2 (5.7%) and grade 3 MR in 1 (2.9%).
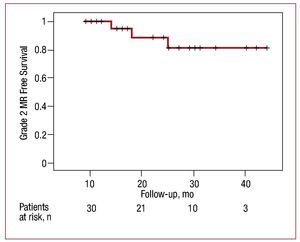
Grade ≥2 MR recurrence-free survival was 95.2% at 15 months and 88.9% at 25 months. Figure 3 shows the Kaplan-Meier survival curve for grade ≥2 MR recurrence over time.
Figure 3. Grade ≥2 mitral regurgitation (MR) recurrence-free survival curve over time.
We found mean LVEF improved significantly: from 40.3% (10.7%) at discharge to 47.7% (15.6%) in late follow-up echocardiography (P=.049). Changes in mean PASP were not statistically significant. Mean transmitral gradient in late follow-up echocardiography was 4.4 (2.1) mmHg and the maximum was 10 (4.4) mmHg.
Preoperative mean left ventricular end-diastole volume was 159.7 (35.7) mL and fell to 123.5 (41.6) mL (P<.001); left ventricular end-systole volume fell from preoperative 89.2 (38.8) mL to 69.4 (37.5) mL in late follow-up (P<.001).
DISCUSSION
The results of our series show the CMA IMR ETlogix ring produces significantly low mid-/long-term incidence of MR recurrence. The excellent durability of this repair technique is shown by the grade ≥2 MR recurrence-free survival of 95.2% at 15 months and 88.9% at 25 months. Similarly, repair durability and the consequent low MR recurrence rate facilitate a regression in left ventricular dilatation.
Bolling et al7 introduced the practice of "overcorrecting" by implanting a completely symmetrical mitral annulus, ie, a ring 1-2 sizes smaller than indicated by the intertrigonal distance. Although this technique led to significantly improved short-term results, more and more groups report it leads to high mid-term MR recurrence.
Kwan et al4 showed the mitral valve deformation pattern is asymmetric in CIMR, but symmetrical in dilated cardiomyopathy. These differences in mitral valve geometry in CIMR underline the importance of the fact that in this entity the P2 and P3 segments present restrictive asymmetric motion also associated with valvular annulus dilatation.
As we reported elsewhere, to achieve our objective of a suitable, homogeneous coaptation surface in patients with CIMR, our repair should affect the entire mitral ring surface (the complete annulus), reduce the anteroposterior distance of the mitral ring more markedly than standard techniques (overcorrective ring), and act by compensating papillary muscle displacement.1
These objectives are more easily achieved thanks to the CMA IMR ETlogix ring, which is specifically designed to act on the geometric alterations produced in CIMR through its innovative three-dimensional asymmetric design.
At the time of writing, only two publications report treatment of CIMR with this asymmetric ring. In 2006, Daimon et al5 presented the first results of using this ring in a multicenter study of 59 patients, with excellent short-term postoperative results. Recently, Filsoufi et al,8 who collaborated in developing the ring, reported 3% grade ≥2+ MR recurrence in a series of 40 patients with 15-34 months follow-up.
Like the aforementioned studies, our results show mitral valve repair with the asymmetric ring produces excellent mid-term results, with durability not only of mitral valve competence but also in the geometric modifications to the mitral valve. Notwithstanding, larger clinical series and longer clinical follow-ups are needed to confirm the durability of this reconstruction technique.
Correspondence: Dr. V.X. Mosquera Rodríguez.
Servicio de Cirugía Cardiaca. Complexo Hospitalario Universitario A Coruña.
As Xubias, 86. 15006 A Coruña. Spain.
E-mail: victor.x.mosquera.rodriguez@sergas.es
Received March 31, 2009.
Accepted for publication October, 2009.