The advent of transcatheter aortic valve implantation has revolutionized the treatment of calcific aortic valve stenosis. Elderly patients who were previously considered inoperable have currently an efficacious and safe therapy that provides better survival. In addition, current practice guidelines tend to recommend earlier intervention to avoid the irreversible consequences of long-lasting pressure overload caused by the stenotic aortic valve. Appropriate timing of the intervention relies significantly on imaging techniques that provide information on the severity of the aortic stenosis as well as on the hemodynamic consequences and cardiac remodeling. While left ventricular ejection fraction remains one of the main functional parameters for risk stratification in patients with severe aortic stenosis, advances in imaging techniques have provided new structural and functional parameters that allow the identification of patients who will benefit from intervention before the occurrence of symptoms or irreversible cardiac damage. Furthermore, ongoing research aiming to identify the medical therapies that can effectively halt the progression of aortic stenosis relies heavily on imaging endpoints, and new imaging techniques that characterize the metabolic activity of calcific aortic stenosis have been proposed to monitor the effects of these therapies. The present review provides an up-to-date overview of the imaging advances that characterizes the pathophysiology and that have changed the management paradigm of aortic stenosis.
Keywords
Over 7 million people in Europe and North America have aortic valve stenosis (AS). Significant AS is the main indication of surgical and/or transcatheter interventions for valvular heart disease in Europe, with the prevalence being expected to rise significantly in the upcoming years as the population becomes older.1,2 Management and indication of aortic valve replacement (AVR) are determined by the presence of signs and symptoms associated with severe AS and the hemodynamic consequences diagnosed with imaging techniques.
Currently, clinical practice guidelines recommend AVR in patients with symptomatic severe AS, and in asymptomatic patients with left ventricular (LV) systolic dysfunction.3–5 The guidelines also consider AVR in patients with asymptomatic severe AS who have markers of poor prognosis such as peak aortic jet velocity> 5 m/s, mean aortic pressure gradient> 60mmHg, and LV ejection fraction <55%.6 Furthermore, a pathologic response to exercise testing, severe LV hypertrophy and increased levels of natriuretic peptides are also considered in decision-making regarding asymptomatic patients with severe AS. Other imaging aspects that are not currently considered in the guidelines but have been associated with the prognosis of patients with severe AS include the presence of impaired myocardial mechanics, as assessed with strain imaging, and myocardial fibrosis, as assessed by cardiovascular magnetic resonance (CMR) techniques. Therefore, cardiac imaging is key in the diagnosis and management of patients with severe AS.7–10
Transthoracic echocardiography is the imaging technique of choice to evaluate the severity and the hemodynamic consequences of AS.11 However, in daily clinical practice, echocardiography often demonstrates contradictory results, challenging the diagnosis of AS severity. Low-dose dobutamine stress echocardiography, as well as cardiac computed tomography (CCT), provide useful information to identify patients with true severe AS.12–15 Furthermore, as the population ages, there will be an increase in the prevalence of cardiac comorbidities that challenge the diagnosis and management when severe AS coexists (ie, cardiac amyloidosis) and in which other imaging techniques such as nuclear imaging or CMR play an important role. Finally, although the pathophysiology of AS has been extensively studied, the ability to image the pathophysiological mechanisms of progression of AS with new molecular imaging techniques has revived research into medical therapies that may potentially halt the natural history of AS.16,17
This review appraises the latest updates in cardiac imaging for the understanding of the pathophysiology of AS and the diagnosis and risk stratification of patients with severe AS. In addition, the role of cardiac imaging in the development of alternative therapies to AVR will be reviewed (figure 1).
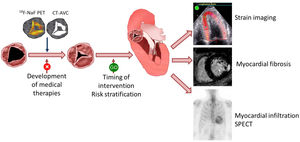
Central illustration. Multimodality imaging in aortic stenosis. In the evaluation of the effect of therapies targeting the pathophysiology underlying the progression of calcific aortic stenosis, imaging techniques such as 18F-NaF positron emission tomography or computed tomography can detect structural changes of the valve earlier than the hemodynamic consequences of the stenosis. Once severe aortic stenosis is diagnosed, markers other than symptoms and left ventricular ejection fraction can help to risk stratify patients who may benefit from early intervention. These markers include myocardial strain imaging, myocardial fibrosis on cardiovascular magnetic resonance and, in some patients, the presence of transthyretin cardiac amyloidosis can be ruled out with the use of technetium 99m pyrophosphate scintigraphy. AVC, aortic valve calcium; CT, computed tomography; F, fluor; NaF, sodium fluorine; PET, positron emission tomography; SPECT, single photon emission computed tomography.
Transthoracic echocardiography is the imaging technique of choice to diagnose the presence and severity of AS. According to current guidelines,4 severe AS is based on peak jet velocity> 4 m/s, mean transvalvular gradient> 40mmHg, and calculated aortic valve area (AVA) <1cm2. However, these measurements have some challenges. AVA calculation by continuity equation is heavily influenced by variations in the measurement of the LV outflow tract. Two-dimensional echocardiography assumes that the LV outflow tract is circumferential instead of oval-shaped, introducing the smallest of the LV outflow tract measurements in the continuity equation, resulting in significant underestimation of the AVA compared with 3-dimensional techniques.18 In addition, peak jet velocity and mean transvalvular gradient are Doppler-based parameters influenced by LV function, the patient́s hemodynamic status, and the correct alignment of the ultrasound beam with the ejection jet through the aortic valve. Consequently, it is well known that 40% of patients with severe AS defined as an AVA <1cm2 may show low gradient (< 40mmHg) due to reduced stroke volume (low flow <35mL/m2) because of reduced LV ejection fraction (classic low flow low gradient severe AS) or because of severe LV hypertrophy and small ventricular cavity (paradoxical low flow low gradient severe AS). In classic low-flow low-gradient severe AS, inducing flow and contractile reserve with low-dose dobutamine stress echocardiography and demonstrating that mean transvalvular gradient increases> 40mmHg while AVA remains <1cm2 helps to establish the diagnosis of true severe AS.12,19 Accordingly, current guidelines include low-dose dobutamine stress echocardiography with a class I recommendation in this clinical scenario. However, the role of low-dose dobutamine stress echocardiography in patients with paradoxical low-flow low-gradient severe AS is less well established. Patients with paradoxical low-flow low-gradient severe AS are mostly women, with a history of hypertension and a small left ventricle with associated significant hypertrophy.20 Clavel et al.21 have proposed the calculation of the projected AVA which takes into consideration the mean transvalvular flow rate, a parameter that varies widely from patient to patient during stress echocardiography. The projected AVA at a normal flow rate showed stronger correlation with the explanted aortic valve weight than peak stress AVA or mean gradient.22 However, it should be noted that, in patients with paradoxical low-flow low-gradient severe AS, low-dose dobutamine stress echocardiography may not be hemodynamically well tolerated and current guidelines do not include this imaging technique for these patients.
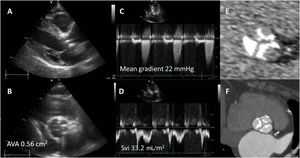
When low-dose dobutamine stress echocardiography is inconclusive and contractile reserve cannot be induced or flow rate normalized, other surrogates of severe AS should be considered. Computed tomography (CT) allows quantification of the calcification burden of the aortic valve. The thickened, hyperechogenic aortic cusps that suggest calcific AS on echocardiography are best visualized with noncontrast CCT. Large, observational studies have shown an association between aortic valve calcium burden and mean transvalvular gradient and AVA.23 Importantly, the cutoff values of aortic valve calcium burden to define severe AS differ between men and women:> 2000 and> 1200, respectively (figure 2).14,15 In the diagnostic algorithm of patients with low-flow low-gradient severe AS, noncontrast CCT is considered to establish the diagnosis and decision making.4
Diagnosis of severe aortic stenosis with computed tomography in a patient with discordant grading on echocardiography. On the parasternal long-axis view (A) and the short-axis view (B) of the aortic valve, thickened, hyperechogenic leaflets can be observed, suggesting severe aortic stenosis. However, on continuous wave Doppler signal through the aortic valve, the mean transvalvular pressure gradient is <40mmHg and on pulsed wave Doppler signal, the calculated stroke volume is <35mL/m2. The calculated aortic valve area is <1cm2. Noncontrast-enhanced computed tomography shows an aortic valve calcium score of 2500 arbitrary units (E) and on contrast-enhanced computed tomography, the valve shows 3 severely calcified leaflets while the thickening cannot be visualized (F). AVA, aortic valve area; Sv, stroke volume index.
More recently, the measurement of aortic valve calcium burden with contrast-enhanced CCT has been validated against noncontrast CCT and explanted aortic valve weights in a group of patients with various grades of AS.24 This imaging technique has superior spatial resolution to noncontrast CCT and allows estimation of the fibrotic component of the aortic valve. Measurement of the fibrocalcific volume of the aortic valve with contrast-enhanced CCT demonstrated a better correlation with peak aortic jet velocity, particularly in women. In addition, the ratio between the fibrotic and the calcific component of the aortic valve decreased with increasing severity of AS. These results are promising since contrast-enhanced CCT is frequently used in patients undergoing transcatheter aortic valve implantation and, in those with low-flow low-gradient severe AS, the use of noncontrast CCT may be obviated.
NEW IMAGING INSIGHTS IN RISK STRATIFICATION OF PATIENTS WITH AORTIC VALVE STENOSISThe progressive reduction in in-hospital mortality after surgical AVR over the years to levels below 3% has been related not only to technical advances but also to the referral of patients probably with less advanced disease and who may even be asymptomatic.25 Appropriate timing of aortic valve intervention is pivotal to improve patient outcomes and reduce the short- and long-term risk of the intervention. Current guidelines recommend aortic valve intervention when AS is severe and causes symptoms or reduction in LV ejection fraction (LVEF).4 However, before severe AS causes symptoms or LVEF falls to <50%, it has been hypothesized that earlier intervention would improve outcomes by avoiding the development of the adverse hemodynamic and structural consequences of severe AS. The results of the RECOVERY and the AVATAR trials demonstrated that asymptomatic patients with severe AS undergoing AVR had better outcomes than those randomized to watchful waiting management and emphasize the need for new markers that can reliably identify patients who will benefit from intervention.26,27
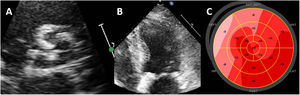
The pressure overload caused by severe AS induces an increase in LV wall stress. To maintain LV systolic function, the LV responds with myocardial hypertrophy characterized by increased muscle fiber diameter and a parallel addition of myofibrils.28 In addition, there is an increase in perivascular fibrosis, interstitial fibrosis and myocyte apoptosis as a consequence of the impaired coronary flow reserve and myocardial ischemia.29 These structural changes appear before there is a reduction in LVEF. However, other imaging parameters have demonstrated to be more sensitive than LVEF in the detection of such changes. Strain imaging techniques and the measurement of myocardial deformation have been demonstrated to be more sensitive than LVEF in the detection of LV systolic dysfunction. Patients with severe AS and LVEF> 50% usually have impaired values of LV global longitudinal strain (LV-GLS) (figure 3). LV-GLS is a more reproducible measurement than LVEF and it has been associated with outcomes in asymptomatic patients with severe AS. Compared with age- and sex-matched controls without AS, patients with asymptomatic severe AS showed more impaired LV-GLS (19.6± 2.1% vs 17.9±2.5%; P <.001, respectively) but similar LVEF (61.5±5.9% vs 62.1±6.3%; P <.001, respectively).30 In addition, the deterioration of LV-GLS further impaired over 12 months of watchful waiting follow-up whereas LVEF remained unchanged.30 These findings suggest that LVEF is a late marker of the consequences of AS on the myocardium. In addition, LV-GLS has incremental prognostic value over LVEF. In an individual patient data-based meta-analysis including patients with severe AS and preserved LVEF, a cutoff value of LV-GLS <14.7% was associated with a 2.5-fold increase in mortality.31 Currently, the guidelines do not include LV-GLS as a basis for AVR decision-making since there are no prospective, randomized trials basing this decision on LV-GLS values.
Evaluation of left ventricular systolic function with strain imaging in aortic stenosis. Example of a patient with calcific aortic stenosis. A: short-axis view of the tricuspid aortic valve, calcified, leading to severe hypertrophy of the left ventricle (B). Despite having normal left ventricular ejection fraction, left ventricular global longitudinal strain is impaired, particularly in the most hypertrophied left ventricular segments (C, septal).
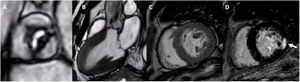
Values of LV-GLS have been correlated with CMR-derived parameters of myocardial reactive and replacement fibrosis.32 Extracellular volume fraction and native and postcontrast T1 mapping values (as surrogates of reactive, interstitial fibrosis) and the presence and mass of late-gadolinium enhancement (reflecting replacement fibrosis) on CMR have been associated with clinical outcomes in patients with AS33 (figure 4). Both reactive (T1 mapping) and replacement fibrosis progress as the AVA decreases and the most rapid progression occurs when AS is severe.34 Increased extracellular volume fraction and T1 mapping values have been associated with adverse LV remodeling and heart failure. However, after AVR, reactive fibrosis regresses and T1 mapping values decrease.35 In contrast, LGE does not disappear once the aortic valve has been replaced. A noninfarct-like pattern of LGE is more frequently observed than an infarct-like pattern in patients with severe AS.36 Right ventricular insertion points, patchy focal and mid-wall myocardial enhancement are the most common locations of noninfarct-like pattern of LGE. The presence of LGE is associated with reduced survival and lack of improvement in clinical symptoms after AVR.37,38 Therefore, it is conceivable that characterization of myocardial fibrosis in AS may help to better define the timing of intervention. Studies such as the EVOLVED trial will clarify the role of CMR in the risk stratification of these patients.39
Cardiovascular magnetic resonance of a patient with severe aortic stenosis. The cine images of the aortic valve (A), the 3-chamber (B) and short-axis (C) views of the left ventricle (B) show the restrictive opening of the aortic valve and the marked left ventricular hypertrophy. D: late gadolinium contrast enhancement of the short-axis of the left ventricle with focal fibrosis in the posterior segment (arrow).
Recently, an association between AS and transthyretin cardiac amyloidosis (ATTR-CA) has been described in various series.40,41 Both diseases are associated with the aging process of the population, and their prevalence increases with age. The deposition of amyloid protein in the interstitial extracellular matrix leads to LV hypertrophy, which may be impossible to differentiate from that caused by AS alone. Red flags increasing the suspicion of ATTR-CA include hypertrophy of the RV and interatrial septum, amarked restrictive diastolic filling pattern of the LV, and thickened mitral and tricuspid valves. Using speckle tracking echocardiography, the presence of a “cherry pattern” with more preserved longitudinal strain in the apical segments of the LV compared with the mid and basal segments also suggests the presence of ATTR-CA but is not specific.42 The increased awareness of ATTR-CA, the advent of effective therapies to halt the disease process and the increased use of technetium 99m pyrophosphate (Tc-99m PYP) scintigraphy as diagnostic gatekeeper, have significantly increased the prevalence of this disease (figure 5). Among patients with severe AS undergoing transcatheter aortic valve implantation, the prevalence of ATTR-CA ranges between 8% and 16% whereas, among patients with cardiac amyloidosis, the prevalence of severe AS is 1.8%.40,43 Without treatment, the association of both diseases has a dismal prognosis.44 However, transcatheter aortic valve implantation has been associated with improved survival.40,41 There remain several unknowns in this conundrum. The true prevalence of ATTR-CA among patients with AS remains unknown because Tc-99m PYP scintigraphy, which is a more sensitive and specific imaging test than CMR or speckle tracking echocardiography, is not systematically performed. The pathophysiological association between both diseases, ATTR-CA and AS, is not fully understood and while both of them are associated with age, there remains the hypothesis that amyloid deposition could be the cause of the cardiomyopathy and AS. Infiltration of the aortic cusps could be a trigger for endothelial damage and calcification.45 Finally, AVR, when indicated, is associated with better survival than medical therapy. However, patients with concomitant ATTR-CA remain at high risk of heart failure hospitalizations after AVR, suggesting the need for close follow-up. Whether disease-modifying therapies for amyloid cardiomyopathy after AVR would lead to better survival remains to be investigated.
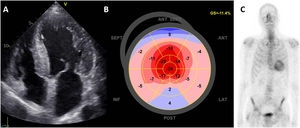
Transthyretin cardiac amyloidosis in a patient with severe aortic stenosis. A: apical 4-chamber view with concentric hypertrophy of the left ventricle, thickened mitral leaflets and dilated atria. On echocardiographic strain analysis, the bull's eye plot of the left ventricle shows apical sparing with more preserved values of longitudinal strain in the apex (B). On bone-scintigraphy, there is uptake by the heart (C). GS, global strain.
Medical therapies targeting the progression of calcific AS to delay AVR have been tested in several randomized clinical trials. These therapies include lipid-lowering therapies, renin-angiotensin-aldosterone system inhibitors, drugs with metabolic targets that promote osteogenesis of the valve.46 However, the results have so far been discouraging since no therapy has been demonstrated to be efficacious. Several hypotheses have been proposed to explain the results of the trials. First, among the patients included in those trials, the disease was possibly too advanced (moderate and severe AS) for medical therapy to be able to halt disease progression, or the disease was at a very early stage (aortic sclerosis or mild AS), which would need very long-term follow-up to demonstrate the efficacy of the medical treatment. In addition, most of the trials used echocardiographic endpoints to assess disease progression and these endpoints consisted of the hemodynamic consequences of the AS (eg, peak jet aortic velocity, mean transvalvular gradient…), which could be influenced by other variables and not only by the aortic valve itself (eg, LVEF, blood systolic pressure…).
In this regard, CT and nuclear techniques have provided new tools to monitor the progression of the calcific process of the aortic valve and could perhaps lead to new imaging endpoints that could show the efficacy of these therapies. Assessment of aortic valve calcium burden with noncontrast-enhanced CT is reproducible and the technique has an excellent scan-rescan repeatability. Furthermore, the amount of calcium in the aortic valve has been associated with the severity of AS and the occurrence of adverse cardiovascular events.14,15,23 This has resulted in the adoption of this imaging technique to monitor the progression of aortic valve calcification and its use as the primary efficacy endpoint of several randomized clinical trials testing novel treatments.47,48 In addition, calcification activity and inflammation of the aortic valve can be assessed with nuclear techniques. 18F-fluorodeoxyglucose (18F-FDG) and 18F-sodium fluoride (18F-NaF) are 2 positron emission tomography (PET) radiotracers that assess, respectively, inflammation and calcification activity49,50 (figure 6). The aortic valve uptake of 18F-NaF correlates with the progression of aortic valve calcium score on noncontrast CT whereas the uptake of 18F-FDG does not.49 Therefore, 18F-NaF PET would be a suitable imaging technique to measure the progression of calcific AS and measure the efficacy of medical therapy. Unfortunately, the potential medical therapies evaluated in randomized clinical trials that have used CT or 18F-NaF PET endpoints have not been shown to be efficacious. The SALTIRE2 trial randomized 150 patients with calcific AS to denosumab or alendronic acid (bone resorption inhibitors) vs placebo and showed that these drugs did not affect disease progression and no differences in change in aortic valve calcium score on CT or in uptake of 18F-NaF on PET were observed across the randomization arms.47 One of the most plausible reasons to explain these findings was that the osteoclasts in the vasculature are nonfunctional and, therefore, any inhibitor targeting osteoclastic resorption (such as bisphosphonates) will not be efficacious in modulating cardiovascular calcification.51
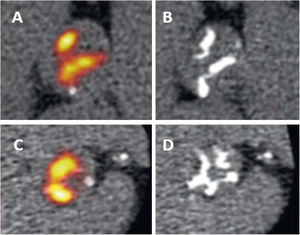
18F-NaF positron emission tomography of 2 patients with calcific aortic stenosis. Panels A and C show the increased valvular 18F-NaF PET uptake at baseline, which predicted progression to macrocalcification on computed tomography after 2 years (B, D). Reproduced with permission from Zheng et al.50.
The AVADEC trial randomized 365 men with calcific AS defined by an aortic valve calcium score ≥ 300 arbitrary units but with a peak aortic jet velocity ≤ 3 m/s to menaquinone-7 vs placebo.48 Menaquinone-7, also known as vitamin K2, is a cofactor involved in the carboxylation of proteins that inhibit arterial calcification. Progression of aortic valve calcification was assessed with noncontrast CT and measurement of the aortic valve calcium score at a 2-year follow-up. There were no significant differences in the changes in aortic valve calcium score between the 2 randomization arms and, therefore, the study concluded that menaquinone-7 did not influence the aortic valve calcification process. Importantly, the trial only included men and the effects of this treatment in women remain unknown. Ongoing randomized clinical trials will provide additional information on the use of these imaging techniques to test the efficacy of medical therapies.46 The radiation burden associated with these techniques will need to be taken into consideration when setting the follow-up of patients under medical therapy.
CONCLUSIONSWhile echocardiography remains the mainstay imaging technique to evaluate patients with calcific AS, the information provided by other imaging techniques allowing the characterization of the myocardial changes induced by the pressure overload and the calcific process of the aortic valve, has opened up a myriad of opportunities to rethink the management of the disease. The ability to detect early structural changes in the LV myocardium that may not be resolved by AVR and may impact negatively on clinical outcomes opens up the possibility to refer patients earlier to AVR. Imaging techniques that allow monitoring of the calcific process of the aortic valve may help to find efficacious medical therapies that will delay or even avoid AVR.
FUNDINGNone.
AUTHORS’ CONTRIBUTIONSE. Ferrer-Sistach: review of the literature, drafting the manuscript, final approval. A. Teis: critical review of the manuscript and final approval, providing illustration advice. A. Bayés-Genís: critical review of the manuscript and final approval. V. Delgado: review of the literature, drafting the manuscript, final approval; illustrations.
CONFLICTS OF INTERESTV. Delgado receives speaker fees from Abbott Vascular, Edwards Lifesciences, Medtronic, Novartis, and GE Healthcare. A. Bayés-Genís has lectured or participated in the advisory boards of Abbott, AstraZeneca, Boehringer-Ingelheim, Novartis, Roche Diagnostics, and Vifor. The remaining authors have nothing to disclose.