Outcome in patients with congenital heart diseases and pulmonary arterial hypertension (PAH) is closely related to right ventricular (RV) function. Two-dimensional echocardiographic parameters, such as strain imaging or RV end-systolic remodeling index (RVESRI) have emerged to quantify RV function.
MethodsWe prospectively studied 30 patients aged 48±12 years with pretricuspid shunt and PAH and investigated the accuracy of multiple echocardiographic parameters of RV function (tricuspid annular plane systolic excursion, tricuspid annular peak systolic velocity, RV systolic-to-diastolic duration ratio, right atrial area, RV fractional area change, RV global longitudinal strain and RVESRI) to RV ejection fraction measured by cardiac magnetic resonance.
ResultsRV ejection fraction <45% was observed in 13 patients (43.3%). RV global longitudinal strain (ρ [Spearman's correlation coefficient]=−0.75; P=.001; R2=0.58; P=.001), right atrium area (ρ=−0.74; P <.0001; R2=0.56; P <.0001), RVESRI (ρ=−0.64; P <.0001; R2=0.47; P <.0001), systolic-to-diastolic duration ratio (ρ=−0.62; P=.0004; R2=0.47; P <.0001) and RV fractional area change (ρ=0.48; P=.01; R2=0.37; P <.0001) were correlated with RV ejection fraction. RV global longitudinal strain, RVESRI and right atrium area predicted RV ejection fraction <45% with the greatest area under curve (0.88; 95%CI, 0.71-1.00; 0.88; 95%CI, 0.76-1.00, and 0.89; 95%CI, 0.77-1.00, respectively). RV global longitudinal strain >−16%, RVESRI ≥ 1.7 and right atrial area ≥ 22 cm2 predicted RV ejection fraction <45% with a sensitivity and specificity of 87.5% and 85.7%; 76.9% and 88.3%; 92.3% and 82.4%, respectively.
ConclusionsRVESRI, right atrial area and RV global longitudinal strain are strong markers of RV dysfunction in patients with pretricuspid shunt and PAH.
Keywords
Pulmonary arterial hypertension (PAH) is a complication of uncorrected or recently repaired congenital heart diseases (CHD).1 PAH in patients with CHD (PAH-CHD) is associated with increased mortality, notably when there is Eisenmenger syndrome.2 PAH-CHD patients have lower life expectancy than patients without PAH.3 Although survival appears to be better than in idiopathic PAH, mortality remains high, especially for patients with a pretricuspid shunt.4 Indeed, heart failure is more often observed compared with patients with posttricuspid shunt, related to different levels of right ventricular (RV) adaptation.5 Follow-up of these patients requires assessment of RV function. Cardiac magnetic resonance (CMR) is recognized as the gold standard for quantification of RV volume and function and has demonstrated its accuracy in the prognosis of PAH.6 In PAH-CHD, CMR provides a wide range of anatomical and functional information, but its routine use is limited by its availability and cost.7 Transthoracic echocardiography is routinely used for diagnosis and follow-up.8 Recent echocardiographic parameters, such as strain imaging or RV end-systolic remodeling index (RVESRI), are new parameters to evaluate RV function and remodeling. RVESRI has recently emerged as a simple, reproducible, and strong prognostic marker in adults with idiopathic, connective-tissue disease, drug and toxins or familial PAH.9 In PAH-CHD, recommendations regarding which echocardiographic parameter of RV function to use are lacking.
The main objective of this study was to assess the reliability of echocardiographic parameters of RV function, including RV strain and RVESRI, to detect RV dysfunction, compared with the gold standard RV ejection fraction (RVEF) measured by CMR in a prospective cohort of patients with pretricuspid shunt and PAH.
METHODSOver 1 year, 30 patients with PAH and pretricuspid shunt (atrial septal defect and/or partial anomalous pulmonary venous return) were prospectively included. Patients underwent CMR, echocardiography and right heart catheterization within 2 days. The study design was approved by an external ethics committee (RCB-2018-A00332-53). All patients gave written informed consent. PAH was classified as Eisenmenger syndrome, as PAH with prevalent systemic-to-pulmonary shunt, or as PAH after defect correction. PAH was defined by a mean pulmonary arterial pressure ≥ 20mmHg, pulmonary vascular resistance ≥ 3 WU, and a left atrial pressure or pulmonary arterial wedge pressure ≤ 15mmHg.10 New York Heart Association-World Health Organization functional class was collected. The following risk scores were calculated for each patient: REVEAL 2.0 score11 and Right Heart score ().12
Cardiac magnetic resonanceCMR studies were performed using a 1.5-Tesla Magnet Discovery MR 450 w (General Electric Healthcare, United States). Functional cine images in long and short-axis views were performed using breath-hold steady state-free precession sequences covering from the base to the apex (repetition time=4.1/ echo time=1.9, Flip angle=15°). Postprocessing was performed on Arterys software (Arterys, United States) for measurement of RV and left ventricular (LV) end-diastole and end-systole volumes and ejection fractions. Normal LV systolic function was defined by a LV ejection fraction ≥ 57%.13 RV dilatation was defined by a RV end-diastolic volume>112 mL/m2 in women and> 121 mL/m2 in men.13
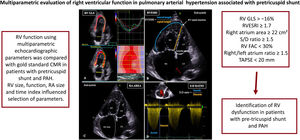
EchocardiographyTransthoracic echocardiography studies were acquired using a Vivid E95 GE (GE Healthcare Vingmed Ultrasound, Norway) using a standardized approach8,14 by 2 certified cardiologists blinded to CMR. Conventional right heart metrics were measured over 3 cycles: M-mode tricuspid annular plane systolic excursion (TAPSE)15; tissue-doppler imaging systolic velocity of the tricuspid valve; systolic-to-diastolic duration ratio (S/D ratio) defined as the ratio between systolic and diastolic tricuspid regurgitation time16,17; and right atrium dilatation defined as end-systolic right atrium area> 18cm2 (figure 1). RV dilatation was defined by a RV end-diastolic area> 11.7cm2/m2 in women and> 12.6cm2/m2 in men.18 Pericardial effusion was noted. Additional parameters of global RV function were measured: RV fractional area change (FAC)15 and RV global longitudinal strain (RVGLS), measured using speckle-tracking imaging.19 RV septal, lateral and apical landmarks were manually positioned in a modified apical 4-chamber view. Automatic function imaging automatically tracked 6 segments from the basal septum to the apex, which were manually adjusted if necessary. The RVESRI was defined as the lateral length/septal height ratio (figure 1).9
Central illustration. Echocardiographic parameters predictive of right ventricular dysfunction. Right ventricular global longitudinal strain (RVGLS) is recorded in apical 4-chamber view, and 6 myocardial segments are automatically tracked. Right ventricular end-systolic remodeling index (RVESRI) is a simple ratio of end-systolic lateral length/septal height. Lateral length is measured from the lateral tricuspid annulus to the insertion point of the RV on the interventricular septum (IVS). The septal height is a straight line from the septal tricuspid annulus to RV insertion on the IVS. RA area is manually contoured in end-systole. The systolic-to-diastolic duration ratio (S/D ratio) is defined as the ratio of systolic-to-diastolic duration of tricuspid regurgitation. Note the large atrial septal defect (red arrow) and dilatation of right heart. CMR, cardiac magnetic resonance; LA, left atrium; LV, left ventricle; PAH, pulmonary arterial hypertension; RA, right atrium; RV, right ventricle; TAPSE, tricuspid annular plane systolic excursion.
Right heart catheterization was performed following a standardized protocol under local anesthesia in spontaneously breathing patients without oxygen support.20 Oxygen consumption was directly measured using a canopy. The Fick method was applied to determine pulmonary cardiac output, the pulmonary to systemic cardiac output ratio, and pulmonary vascular resistance, expressed in Wood units.
Statistical analysisContinuous variables are expressed as mean±standard deviation or median [interquartile range], as appropriate. The normality of the distribution was tested with the Shapiro-Wilk test. Comparisons between measurements in patients with RVEF <45% vs ≥ 45% were performed. A paired t-test was used when variables were normally distributed, and the homogeneity of variance was checked by the Levene test. Otherwise, a nonparametric Mann-Whitney test was used. Categorical data were compared using a chi-square test or Fisher exact test. The agreement between CMR results and echocardiography parameters was investigated assessing their linear relationships. The strength of correlations was assessed with the Spearman test and reported as ρ. Pearson is reported for correlation graphs and ρ for monotonic ranked associations. Receiver operator characteristic (ROC) curves were created for each relevant echocardiography variable to assess RVEF and the area under the curve (AUC) was reported. The best discriminatory value was determined to provide the best-case sensitivity and specificity of the variable for various RVEF thresholds.13,21 Relevant investigated echocardiography parameters included the 4 prognostic markers described in patients with Eisenmenger syndrome: right atrial area, right atrial/left atrial area ratio, S/D ratio, and TAPSE.22 Two-dimensional echocardiographic parameters of RV function were also measured including RVGLS, RVFAC, and RVESRI.9 To assess the interobserver reproducibility of RVESRI measurements, a linear regression analysis and assessment of the mean bias by the Bland-Altman analysis were performed. For all analyses, a threshold of α=0.05 was chosen for statistical significance. The most relevant parameters were combined in a nonweighted score as an exploratory analysis. Statistical analyses were performed using Stata software, version 11.2 (StataCorp, United States).
RESULTSPatient populationThirty patients were included (table 1). CMR and catheterization measurements are reported in table 2. PAH was severe as evidenced by a mean pulmonary artery pressure of 50±13mmHg and pulmonary vascular resistance of 9.4 WU [5.1-15.0].
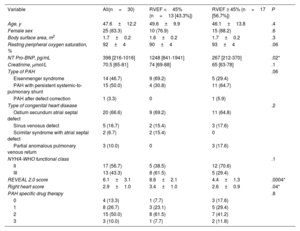
Patients’ baseline characteristics.
| Variable | All(n=30) | RVEF <45% (n=13 [43.3%]) | RVEF ≥ 45% (n=17 [56.7%]) | P |
|---|---|---|---|---|
| Age, y | 47.6±12.2 | 49.6±9.9 | 46.1±13.8 | .4 |
| Female sex | 25 (83.3) | 10 (76.9) | 15 (88.2) | .6 |
| Body surface area, m2 | 1.7±0.2 | 1.6±0.2 | 1.7±0.2 | .3 |
| Resting peripheral oxygen saturation, % | 92±4 | 90±4 | 93±4 | .06 |
| NT Pro-BNP, pg/mL | 398 [216-1016] | 1248 [841-1941] | 267 [212-370] | .02* |
| Creatinine, μmol/L | 70.5 [65-81] | 74 [69-88] | 65 [63-78] | .1 |
| Type of PAH | .06 | |||
| Eisenmenger syndrome | 14 (46.7) | 9 (69.2) | 5 (29.4) | |
| PAH with persistent systemic-to-pulmonary shunt | 15 (50.0) | 4 (30.8) | 11 (64.7) | |
| PAH after defect correction | 1 (3.3) | 0 | 1 (5.9) | |
| Type of congenital heart disease | .2 | |||
| Ostium secundum atrial septal defect | 20 (66.6) | 9 (69.2) | 11 (64.8) | |
| Sinus venosus defect | 5 (16.7) | 2 (15.4) | 3 (17.6) | |
| Scimitar syndrome with atrial septal defect | 2 (6.7) | 2 (15.4) | 0 | |
| Partial anomalous pulmonary venous return | 3 (10.0) | 0 | 3 (17.6) | |
| NYHA-WHO functional class | .1 | |||
| II | 17 (56.7) | 5 (38.5) | 12 (70.6) | |
| III | 13 (43.3) | 8 (61.5) | 5 (29.4) | |
| REVEAL 2.0 score | 6.1±3.1 | 8.6±2.1 | 4.4±1.3 | .0004* |
| Right heart score | 2.9±1.0 | 3.4±1.0 | 2.6±0.9 | .04* |
| PAH specific drug therapy | .8 | |||
| 0 | 4 (13.3) | 1 (7.7) | 3 (17.6) | |
| 1 | 8 (26.7) | 3 (23.1) | 5 (29.4) | |
| 2 | 15 (50.0) | 8 (61.5) | 7 (41.2) | |
| 3 | 3 (10.0) | 1 (7.7) | 2 (11.8) |
NYHA-WHO, New York Heart Association-World Health Association; PAH, pulmonary arterial hypertension; RVEF, right ventricular ejection fraction.
Values are expressed as No. (%), mean±standard deviation, or median [interquartile range].
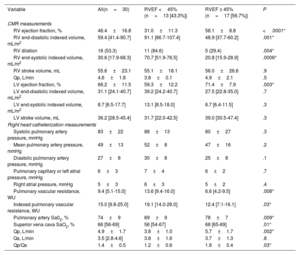
Cardiovascular magnetic resonance and hemodynamic parameters according to right ventricular ejection fraction.
| Variable | All(n=30) | RVEF <45% (n=13 [43.3%]) | RVEF ≥ 45% (n=17 [56.7%]) | P |
|---|---|---|---|---|
| CMR measurements | ||||
| RV ejection fraction, % | 46.4±16.8 | 31.0±11.3 | 58.1±8.8 | <.0001* |
| RV end-diastolic indexed volume, mL/m2 | 59.4 [41.4-90.7] | 91.1 [86.7-107.4] | 48.9 [37.7-60.2] | .001* |
| RV dilation | 16 (53.3) | 11 (84.6) | 5 (29.4) | .004* |
| RV end-systolic indexed volume, mL/m2 | 30.6 [17.9-68.3] | 70.7 [51.9-76.5] | 20.8 [15.9-28.9] | .0006* |
| RV stroke volume, mL | 55.6±23.1 | 55.1±18.1 | 56.0±26.6 | .9 |
| Qp, L/min | 4.6±1.8 | 3.8±0.1 | 4.9±2.1 | .5 |
| LV ejection fraction, % | 66.2±11.5 | 59.3±12.2 | 71.4±7.9 | .003* |
| LV end-diastolic indexed volume, mL/m2 | 31.1 [24.1-40.7] | 39.2 [24.2-40.7] | 27.5 [22.8-35.0] | .7 |
| LV end-systolic indexed volume, mL/m2 | 8.7 [6.5-17.7] | 13.1 [8.5-18.0] | 6.7 [6.4-11.5] | .3 |
| LV stroke volume, mL | 36.2 [28.5-45.4] | 31.7 [22.0-42.5] | 39.0 [30.5-47.4] | .3 |
| Right heart catheterization measurements | ||||
| Systolic pulmonary artery pressure, mmHg | 83±22 | 88±13 | 80±27 | .3 |
| Mean pulmonary artery pressure, mmHg | 49±13 | 52±8 | 47±16 | .2 |
| Diastolic pulmonary artery pressure, mmHg | 27±8 | 30±8 | 25±8 | .1 |
| Pulmonary capillary or left atrial pressure, mmHg | 6±3 | 7±4 | 6±2 | .7 |
| Right atrial pressure, mmHg | 5±3 | 6±3 | 5±2 | .4 |
| Pulmonary vascular resistance, WU | 9.4 [5.1-15.0] | 13.6 [9.4-16.0] | 6.6 [4.2-9.5] | .008* |
| Indexed pulmonary vascular resistance, WU | 15.0 [9.8-25.0] | 19.1 [14.0-26.0] | 12.4 [7.1-16.1] | .03* |
| Pulmonary artery Sa02, % | 74±9 | 69±9 | 78±7 | .009* |
| Superior vena cava SaO2, % | 66 [56-69] | 56 [54-67] | 68 [65-69] | .01* |
| Qp, L/min | 4.9±1.7 | 3.8±1.0 | 5.7±1.7 | .002* |
| Qs, L/min | 3.5 [2.8-4.6] | 3.8±1.6 | 3.7±1.3 | .8 |
| Qp/Qs | 1.4±0.5 | 1.2±0.6 | 1.6±0.4 | .03* |
CMR, cardiac magnetic resonance; LV, left ventricle; Qp, pulmonary output; Qs, systemic output; RV, right ventricle; RVEF, right ventricular ejection fraction; WU, Wood unit.
Values are expressed as No. (%), mean±standard deviation, median [interquartile range].
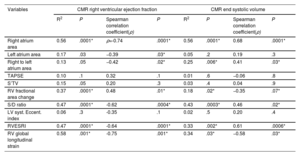
Mean CMR-derived RVEF was 46.4±16.8%, and 13 patients (43.3%) had RVEF <45%. Patients with RVEF <45% had higher risk score (REVEAL 2.0, P=.0004 and right heart score P=.04), higher pulmonary vascular resistance (P=.008), larger RV end-diastolic and end-systolic volumes (P=.001; P=.0006) and larger right atrium (P=.0001). Eight patients with LV dysfunction also had RV dysfunction (P <.05). Peripheral oxygen saturation was correlated with RVEF (ρ=0.39, P=.03; R2=0.20, P <.01). Table 3 shows the comparison between CMR-derived RVEF and echocardiography parameters. Spearman correlations between echocardiography parameters, CMR-RVEF and CMR end-systolic volumes are reported in table 4, and linear regression analysis are reported in figure 2 and figure 3.
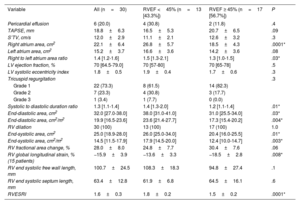
Echocardiographic parameters according to right ventricular ejection fraction.
| Variable | All (n=30) | RVEF <45% (n=13 [43.3%]) | RVEF ≥ 45% (n=17 [56.7%]) | P |
|---|---|---|---|---|
| Pericardial effusion | 6 (20.0) | 4 (30.8) | 2 (11.8) | .4 |
| TAPSE, mm | 18.8±6.3 | 16.5±5.3 | 20.7±6.5 | .09 |
| S’TV, cm/s | 12.0±2.9 | 11.1±2.1 | 12.6±3.2 | .3 |
| Right atrium area, cm2 | 22.1±6.4 | 26.8±5.7 | 18.5±4.3 | .0001* |
| Left atrium area, cm2 | 15.2±3.7 | 16.6±3.6 | 14.2±3.6 | .08 |
| Right to left atrium area ratio | 1.4 [1.2-1.6] | 1.5 [1.3-2.1] | 1.3 [1.0-1.5] | .03* |
| LV ejection fraction, % | 70 [64.5-79.0] | 70 [57-80] | 70 [65-78] | .5 |
| LV systolic eccentricity index | 1.8±0.5 | 1.9±0.4 | 1.7±0.6 | .3 |
| Tricuspid regurgitation | .3 | |||
| Grade 1 | 22 (73.3) | 8 (61.5) | 14 (82.3) | |
| Grade 2 | 7 (23.3) | 4 (30.8) | 3 (17.7) | |
| Grade 3 | 1 (3.4) | 1 (7.7) | 0 (0.0) | |
| Systolic to diastolic duration ratio | 1.3 [1.1-1.4] | 1.4 [1.3-2.0] | 1.2 [1.1-1.4] | .01* |
| End-diastolic area, cm2 | 32.0 [27.0-38.0] | 38.0 [31.0-41.0] | 31.0 [25.5-34.0] | .03* |
| End-diastolic area, cm2/m2 | 19.9 [16.5-23.6] | 23.6 [21.4-27.7] | 17.3 [15.4-20.2] | .004* |
| RV dilation | 30 (100) | 13 (100) | 17 (100) | 1.0 |
| End-systolic area, cm2 | 25.0 [18.9-28.0] | 26.0 [25.0-34.0] | 20.4 [16.0-25.5] | .01* |
| End-systolic area, cm2/m2 | 14.5 [11.5-17.9] | 17.9 [14.5-20.0] | 12.4 [10.0-14.7] | .003* |
| RV fractional area change, % | 28.0±8.0 | 24.8±7.7 | 30.4±7.6 | .06 |
| RV global longitudinal strain, % (15 patients) | −15.9±3.9 | −13.6±3.3 | −18.5±2.8 | .008* |
| RV end systolic free wall length, mm | 100.7±24.5 | 108.3±18.3 | 94.8±27.4 | .1 |
| RV end systolic septum length, mm | 63.4±12.8 | 61.9±6.8 | 64.5±16.1 | .6 |
| RVESRI | 1.6±0.3 | 1.8±0.2 | 1.5±0.2 | .0001* |
LV, left ventricle; RV, right ventricle; RVEF, right ventricular ejection fraction; RVESRI, right ventricle end-systolic remodeling index; S’TV, systolic velocity of the tricuspid valve; TAPSE, tricuspid annular plane systolic excursion.
Values are expressed as No (%) mean±standard deviation, or median [interquartile range]
Relationships between echocardiographic parameters and CMR-derived right ventricular ejection fraction.
| Variables | CMR right ventricular ejection fraction | CMR end systolic volume | ||||||
|---|---|---|---|---|---|---|---|---|
| R2 | P | Spearman correlation coefficient(ρ) | P | R2 | P | Spearman correlation coefficient(ρ) | P | |
| Right atrium area | 0.56 | .0001* | ρ=-0.74 | .0001* | 0.56 | .0001* | 0.68 | .0001* |
| Left atrium area | 0.17 | .03 | −0.39 | .03* | 0.05 | .2 | 0.19 | .3 |
| Right to left atrium area | 0.13 | .05 | −0.42 | .02* | 0.25 | .006* | 0.41 | .03* |
| TAPSE | 0.10 | .1 | 0.32 | .1 | 0.01 | .6 | −0.06 | .8 |
| S’TV | 0.15 | .05 | 0.20 | .3 | 0.03 | .4 | 0.04 | .9 |
| RV fractional area change | 0.37 | .0001* | 0.48 | .01* | 0.18 | .02* | −0.35 | .07* |
| S/D ratio | 0.47 | .0001* | -0.62 | .0004* | 0.43 | .0003* | 0.46 | .02* |
| LV syst. Eccent. index | 0.06 | .3 | -0.35 | .1 | 0.02 | .5 | 0.20 | .4 |
| RVESRI | 0.47 | .0001* | -0.64 | .0001* | 0.33 | .002* | 0.61 | .0006* |
| RV global longitudinal strain | 0.58 | .001* | -0.75 | .001* | 0.34 | .03* | −0.58 | .03* |
CMR, cardiac magnetic resonance; LV, left ventricle; S/D ratio, systolic-to-diastolic duration ratio; S’TV, systolic velocity of the tricuspid valve; TAPSE, tricuspid annular plane systolic excursion; RV, right ventricle; RVEF, right ventricular ejection fraction; RVESRI, right ventricle end-systolic remodeling index.
R2 indicates coefficient of determination by linear regression analysis, with its degree of significance. ρ indicates Spearman correlation coefficient with its degree of significance.
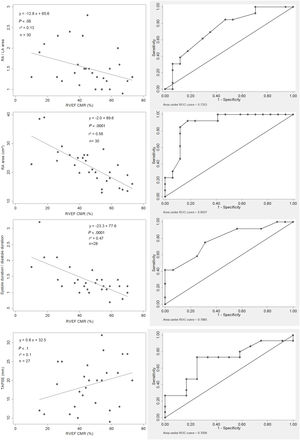
Conventional echocardiographic parameters and RVEF. Correlations between echocardiographic parameters and RVEF (left images). ROC curve (right images): right atrium area ≥ 22cm2 (sensitivity 92.3%; specificity 82.4%), S/D ratio ≥ 1.5 (sensitivity 41.5%, specificity 70.6%), TAPSE <20mm (sensitivity 73.3%, specificity 75%) and right atrium/left atrium ratio ≥ 1.5 (sensitivity 61.5%; specificity 70.6%) identified RV dysfunction. AUC, area under curve; CMR, cardiac magnetic resonance; LA, left atrium; RA, right atrium; RA/LA ratio, right-to-left atrial area ratio; RV, right ventricle; RVEF, right ventricular ejection fraction; S/D ratio, systolic-to-diastolic duration ratio; TAPSE, tricuspid annular plane excursion.
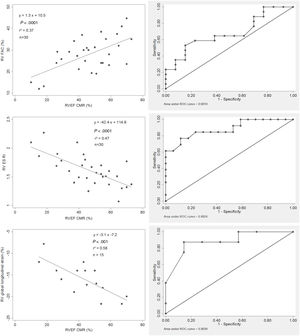
Multidimensional echocardiographic parameters and RVEF. Correlations between echocardiographic parameters and RVEF (left images). ROC curves (right images): RVGLS> −16% (sensitivity 87.5%; specificity 85.7%), RVESRI ≥ 1.7 (sensitivity 76.9%; specificity 88.3%) and RVFAC <30% (sensitivity 58.8%; specificity 76.9%) predicted RV dysfunction. AUC, area under curve; CMR, cardiac magnetic resonance; RVEF, right ventricular ejection fraction; RVESRI, right ventricular end-systolic remodeling index; RVFAC, right ventricular fractional area change; RVGLS, right ventricular global longitudinal strain.
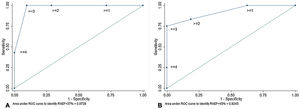
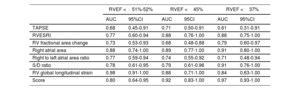
On ROC curves, RVGLS, RVESRI and right atrium area demonstrated the greatest AUC to identify RVEF <45% (AUC=0.88, 95%CI, 0.71-1.00; AUC=0.88, 95%CI, 0.76-1.00 and AUC=0.89, 95%CI, 0.77-1.00, respectively). Right atrium/left atrium ratio, S/D ratio, TAPSE and RVFAC showed fair AUC (AUC=0.74, 95%CI, 0.55-0.92; AUC=0.79, 95%CI, 0.61-0.96; AUC=0.71, 95%CI, 0.50-0.91 and AUC=0.68, 95%CI, 0.48-0.88). The best discriminatory values to predict RVEF <45% were RVGLS> −16% (sensitivity 87.5% and specificity 85.7%); RVESRI ≥ 1.7 (sensitivity 76.9% and specificity 88.3%); RVFAC <30% (sensitivity 58.8% and specificity 76.9%); right atrium area ≥ 22cm2 (sensitivity 92.3% and specificity 82.4%); right atrium/left atrium ratio ≥ 1.5 (sensitivity 61.5% and specificity 70.6%); S/D ratio ≥ 1.5 (sensitivity 41.7% and specificity 93.8%) and TAPSE <20mm (sensitivity 73.3% and specificity 75.0%) (figure 1, figure 2, and figure 3). The AUC of echocardiographic parameters to predict RVEF <37%21, RVEF <45% and RVEF ≤ 51 in women ≤ 52 in men13 are reported in table 5. A score was built with 1 point added if RVESRI ≥ 1.7, RVFAC <30%, right atrium area ≥ 22cm2 and S/D ratio ≥ 1.5. The score was correlated with RVEF (ρ=−0.74, P <.0001; R2=0.64, P <.0001). AUC to identify RVEF <37%21 was 0.97; 95%CI, 0.93-1.00. A score ≥ 3 had a sensitivity of 100% and a specificity of 90.5% to identify RVEF <37% (figure 4A). AUC to identify RVEF <45% was 0.92; 95%CI, 0.83-1.00. A score ≥ 3 had a sensitivity of 75% and a specificity of 100% to identify RVEF <37% (figure 4B).
Receiver operating characteristic curve of echocardiographic parameters*.
| RVEF <51%-52% | RVEF <45% | RVEF <37% | ||||
|---|---|---|---|---|---|---|
| AUC | 95%CI | AUC | 95CI | AUC | 95%CI | |
| TAPSE | 0.68 | 0.45-0.91 | 0.71 | 0.50-0.91 | 0.61 | 0.31-0.91 |
| RVESRI | 0.77 | 0.60-0.94 | 0.88 | 0.76-1.00 | 0.88 | 0.75-1.00 |
| RV fractional area change | 0.73 | 0.53-0.93 | 0.68 | 0.48-0.88 | 0.79 | 0.60-0.97 |
| Right atrial area | 0.88 | 0.74-1.00 | 0.89 | 0.77-1.00 | 0.91 | 0.80-1.00 |
| Right to left atrial area ratio | 0.77 | 0.59-0.94 | 0.74 | 0.55-0.92 | 0.71 | 0.48-0.94 |
| S/D ratio | 0.78 | 0.61-0.95 | 0.79 | 0.61-0.96 | 0.91 | 0.76-1.00 |
| RV global longitudinal strain | 0.98 | 0.91-1.00 | 0.88 | 0.71-1.00 | 0.84 | 0.63-1.00 |
| Score | 0.80 | 0.64-0.95 | 0.92 | 0.83-1.00 | 0.97 | 0.93-1.00 |
AUC, area under the curve; 95CI, 95% of confidence interval; S/D ratio, systolic-to-diastolic duration ratio; TAPSE, tricuspid annular plane systolic excursion; RV, right ventricle; RVEF, right ventricular ejection fraction; RVESRI, right ventricle end-systolic remodeling index.
Receiver operating characteristic curve of echocardiographic parameters to predict RVEF <37, RVEF <45 and RVEF ≤ 51 in women and ≤ 52 in men.
A score was built with 1 point added if RVESRI>=1.7, RVFAC <30%, right atrium area ≥ 22cm2 and S/D ratio ≥ 1.5. AUC to predict RVEF <37% was 0.97; 95%CI, 0.93-1.00. A score ≥ 3 had a sensitivity of 100% and a specificity of 90.5% to identify RVEF <37% (A). AUC to identify RVEF <45% was 0.92; 95%CI, 0.83-1.00. A score ≥ 3 had a sensitivity of 75% and a specificity of 100% to identify RVEF <37% (B). 95%CI, 95% confidence interval; AUC, area under the curve; RVEF: right ventricular ejection fraction; RVESRI, right ventricular end-systolic remodeling index; RVFAC, right ventricular fractional area change.
Interobserver reproducibility of RVESRI parameters was good with a variation coefficient of 4.4% and a mean bias of 0.02±0.25 (). Bias tended to be higher in higher RVESRI values.
Relationship of RVESRI with risk scoreRVESRI was significantly correlated with the right heart score (P=.008) but not REVEAL 2.0 scores.
Feasibility of RV echocardiographic parametersRVESRI and all conventional parameters were measurable in all patients. RVFAC was successfully measured in 96.7% of cases. For RVGLS, RV segments were not automatically detected in 11 patients (36.7%).
DISCUSSIONThis single center prospective study of 30 patients with PAH secondary to pretricuspid shunt compared echocardiographic RV metrics of function and remodeling to CMR. RVFAC, S/D ratio, right atrium area and RVESRI were the most relevant parameters to assess RV function. A score combining RVESRI, right atrium area, S/D ratio and RVFAC was strongly correlated with RVEF and strongly predicted RVEF <37%, a threshold that has been identified as relevant for risk stratification in PAH.21 We support the interest of the RVESRI9 to assess RV function in PAH-CHD patients. Few data on RV echocardiographic evaluation are available in PAH-CHD, mainly because of the heterogeneity of heart defects.5,22,23 To our knowledge, no study has evaluated echocardiographic parameters of RV function compared with the gold standard CMR-RVEF in PAH-CHD patients, as has been done for other CHD.24 An echocardiographic prognostic score was proposed by Moceri et al.22 from a large but heterogeneous cohort of 181 patients with Eisenmenger syndrome. The strength of this study was to demonstrate that conventional echocardiographic parameters eg, TAPSE <15mm, S/D ratio ≥ 1.5, right atrium area ≥ 25cm2, and right atrium/left atrium ratio ≥ 1.5 were predictive of mortality. However, CMR was not available and only 29 patients (16%) had a pretricuspid shunt.22
Conventional echocardiographic RV evaluationTAPSE is a simple, widely used, and strong independent parameter correlated with RVEF in patients with PAH.15,25 In PAH-CHD, TAPSE was associated with adverse outcome and used as a prognostic parameter.22,23 However, it has been suggested that only serial changes in TAPSE were predictive for mortality in this population.26 In our study, TAPSE was not significantly correlated with RVEF. Nonetheless TAPSE <20mm predicted RV dysfunction. This study illustrates some limits of TAPSE. Although it does not appear to be influenced by the type of shunt in patients with Eisenmenger syndrome,5 the presence of a persistent left-to-right shunt and therefore a RV preload in half of our patients may have influenced our results. Moreover, TAPSE is a longitudinal RV function marker. It reflects neither RV remodeling nor RV transverse function, which are impaired in patients with pretricuspid shunt.27
As the RV becomes progressively dysfunctional in PAH-CHD, systole duration increases while diastole shortens. A higher S/D ratio reflects a prolonged RV ejection time for a given stroke volume and a worse RV global performance. Thus, the S/D ratio is a simple tool to assess RV function and is associated with worse prognosis in PAH-CHD.16,17 The S/D ratio was feasible in all patients as they always had tricuspid regurgitation. It was significantly higher in patients with RV dysfunction with a strong correlation with CMR-RVEF. S/D ratio ≥ 1.5 was predictive of RV dysfunction in our study. This cutoff was previously associated with mortality.22
Other parameters have been reported to be predictors of mortality in Eisenmenger syndrome, such as right atrium dilatation assessed by right atrium area or the right atrium/left atrium area ratio.22 These are simple and reliable markers of RV diastolic dysfunction related to RV hypertrophy and wall thickening, which is the initial response to PAH.28 Long-standing shunt at the atrial level in patients with pretricuspid shunt contributes to right atrium dilatation.5 In our study, right atrium area was a good marker of RV function and right atrium area ≥ 22cm2 predicted RV dysfunction. This value approached the 25cm2 cutoff reported to be associated with mortality.22
Incremental value of 2-dimensional echocardiographic evaluation of RV functionThe transverse component of RV systolic function seems to better reflect the global contraction.15 RVFAC is a simple 2-dimensional way to assess both the longitudinal and the transverse components of RV contraction and is correlated with CMR-RVEF in PAH.15,29 In our study, RVFAC exhibited a good correlation with CMR-RVEF. Compared with other types of PAH, patients with Eisenmenger syndrome and posttricuspid shunt have better RVFAC, probably because of preserved RV transverse function.30 On the other hand, RVFAC seems to be more compromised in patients with pretricuspid shunt, probably related to the different and delayed RV adaptation.5 Similarly, in patients with Tetralogy of Fallot characterized by RV volumetric overload and dilatation, RVFAC was highly correlated with CMR-RVEF while 1-dimensional variables, such as TAPSE, were not.31 RVFAC can be susceptible to errors in measurement and combined with other indices can balance these errors in measurement.
Among 2-dimensional parameters, strain imaging using the speckle-tracking technique allows measurement of regional and global deformation of the myocardium. Increased wall tension secondary to higher afterload reduces myocytes shortening and strain. Greater RV wall thickness allowed good automatic myocardial detection with speckle-tracking in patients with PAH. Nevertheless, the feasibility of RVGLS was limited by RV dilatation. In such cases, not all RV segments fitted in the ultrasound field, as previously observed in patients with Tetralogy of Fallot.24 Comparing the performance of RVGLS with other parameters is thus limited by a different and smaller sample. Moreover, discrepancies in measurement methods have been identified.19 In our cohort, we included interventricular septal strain as a component of RVGLS, which reflects not only RV but also LV contractile function. Current ASE/EACVI guidelines suggest> −20% as an abnormal value threshold for RV free wall strain in the general population.8 When RVGLS was feasible, it was strongly correlated with RVEF in our cohort and a cutoff at −16% was predictive of RV dysfunction. In PAH, RVGLS was associated with mortality.19 Moreover CMR determined that RV strain was correlated with ventricular arterial coupling and arterial load.32 Cardiac remodeling is different in patients with Eisenmenger syndrome compared with other types of PAH, with a similar RV longitudinal strain but a higher RV transverse strain.33 However, transverse RV strain is lower in patients with pretricuspid vs posttricuspid shunt, suggesting an altered RV short-axis function with adverse RV remodeling.27 Indeed, the RV in patients with posttricuspid shunt faces higher pressures since birth, leading to modified myocardial fiber orientation also called “fetal phenotype”.34
To assess the complex shape of the RV and its remodeling in patients with PAH, the RVESRI has recently been developed.9 RVESRI incorporates both the longitudinal component of RV adaptation and the end-systolic dimension. This index, measured in end-systole, when the RV wall-stress is the highest in PAH, has been shown to be a strong prognostic marker of clinical worsening in adults with PAH.9 In children, RVESRI was increased in patients with PAH compared with controls and was associated with hemodynamic variables.35 RVESRI was feasible in all patients and interobserver reproducibility was very good, removing the difficulty of assessing the free wall of dilated RV in diastole. In our study, RVESRI was strongly correlated with RV dysfunction. RVESRI was higher in the RV dysfunction group and its mean value placed our population in the worst zone of RV adaptation proposed by Amsallem et al.9 This index does not seem suitable for posttricuspid shunt, in which the presence of a ventricular septal defect does not allow full measurement of septum length.
Patients with pretricuspid shunt and PAH have an unfavorable RV physiology and adaptation with higher mortality.4,36 PAH develops later in life than posttricuspid shunt, with a RV less able to adapt to higher pressures, and RV dysfunction has a prognostic significance in these patients.4,36 Therefore, 2-dimensional echocardiographic assessment is crucial in these patients to accurately evaluate the efficacy of PAH-specific drugs and to offer them an adapted transplant program if needed, classically based on heart and lung transplant, but also double lung transplant with shunt closure.
LimitationsOur single center study has several limitations. First, the number of patients was relatively low because we selected a cohort of patients with pretricuspid shunt and PAH who had a CMR and a right heart catheterization. However, the study was able to demonstrate a significant relationships between echocardiographic markers and RV function. Moreover, to date, no study has compared echocardiographic RV function to the CMR gold standard. We did not study RV 3-dimensional echocardiography although it was feasible to assess RV function. However, similarly to RVGLS, the feasibility of 3-dimensional echocardiography is limited in patients with dilated RV and is operator-dependent, requiring training and a learning curve.31
In building a multiparameter score, it is important to select complementary and reproducible indices. In our exploratory analysis, we combined indices of RV size and function, right atrial size, and time indices. In the future, in a larger cohort, a weighted score could be developed combining the most reproducible and predictive indices for practice accounting for technical variability in measurement. Moreover, this weighted score could be evaluated with outcome prediction.
CONCLUSIONSOur study demonstrates that RVESRI, right atrium area and RVGLS are strong markers of RV dysfunction in patients with pretricuspid shunt and PAH. The prognostic value of these parameters in this population remains to be studied.
FUNDINGS. Hascoet: “Heart Failure Research Grant” from the French Society of Cardiology.
S. Hascoet and E. Valdeolmillos: “Research team Grant” from the Federation Française de Cardiologie.
AUTHORS’ CONTRIBUTIONSE. Fournier, M. Selegny, E. Valdeolmillos and S. Hascoet designed, drafted and revised the manuscript. E. Fournier, M. Selegny, M.A. Isorni, A. Azarine and S. Hascoet contributed to data collection. S. Hascoet performed the statistical analysis. M. Amsallem, F. Haddad, E. Belli, S. Cohen, D. Montani, J. Le Pavec, A. Azarine, E. Fadel, M. Humbert, O. Sitbon, X. Jais, L. Savale, J. Zoghbi and M. A. Isorni revised the manuscript and participated in the final approved version of the manuscript.
CONFLICTS OF INTERESTNone.
AcknowledgementsThe authors would like to thank Florence Lecerf, and Antoinette Wolfe for their contribution to this work.
Pulmonary arterial hypertension is an unusual and dreaded complication of atrial septal defect. Right ventricular function is a key prognosis marker.
Two-dimensional echocardiographic parameters have been developed for assessment of the right ventricle, such as strain and the remodeling index. However, few data are available on their performance in these patients.
WHAT DOES THIS STUDY ADD?Right ventricular global longitudinal strain, right ventricle end-systolic remodeling index and right atrial area are the most reliable echocardiographic parameters of right ventricular ejection fraction in patients with pretricuspid shunt and pulmonary arterial hypertension.
These 2-dimensional parameters might improve risk stratification in these patients.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2022.07.010