Keywords
INTRODUCTION
Amiodarone pulmonary toxicity (APT) is difficult to diagnose because the clinical findings, laboratory results and symptoms are non-specific.1 At present there is no diagnostic tool available to confirm and assess APT activity.
Serum KL-6 (Krebs von den Lungen-6)2 is a glycoprotein expressed in type 2 alveolar pneumocytes that has been recognized as a marker for the activity of interstitial lung disease; however, its role as an early indicator of APT has not been proven.
We describe a patient who developed lung biopsy-proven APT who improved after discontinuation of the drug. Serum KL-6 levels were measured at the onset of clinical symptoms and again after withdrawal of amiodarone.
CASE STUDY
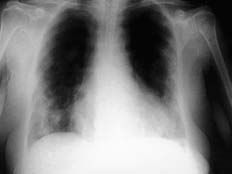
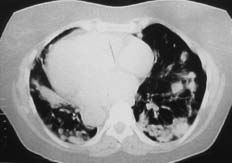
A 69-year-old woman receiving indapamide for hypertension, amiodarone (200 mg/day, cumulative dose of 219 g) for a 3-year history of paroxysmal atrial fibrillation, and dicoumarol derivatives for anticoagulation, presented with a 2-month history of progressive dyspnea on moderate exertion, accompanied by episodes of dry cough, with no chest pain, fever, constitutional symptoms, or other alterations and was admitted to the hospital. The examination revealed the presence of bilateral dry crepitant rales in the lung bases and grade II/VI systolic murmur in the mitral area. The radiological findings (Figures 1 and 2) showed an alveolar-interstitial pattern predominantly in the middle fields and bases, and multiple peripheral pulmonary nodules with poorly defined contours.
Figure 1. Baseline chest x-ray, showing a bilateral pulmonary process predominating in the middle fields and bases, consisting of an interstitial-alveolar pattern with poorly-defined nodules.
Complete blood tests, arterial blood gases and immunological and infectious analyses were performed, with normal results. A slight alteration in thyroid function was detected (TSH, 6.6 U/mL; free T4, 1.3). Amiodarone and desethylamiodarone levels were 0.9 µg/mL and 0.8 µg/mL, respectively. The respiratory function tests showed a moderate restrictive pattern, with a forced vital capacity of 66% and Tiffeneau index of 97%. The carbon monoxide diffusion test was at the lower limit of normality (87%).
Figure 2. High-resolution computed tomography of the chest. A predominantly alveolar pattern is observed, with multiple peripheral pulmonary nodules showing poorly-defined contours, some with cavitation, accompanied by other areas of pulmonary consolidation with an air bronchogram. Although less evident, areas of fibrosis were detected.
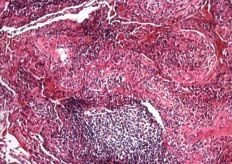
Figure 3. Lung biopsy. Features of organizing pneumonia, lymphoid follicles and alveolar macrophages in septal fibrosis are observed.
On lung biopsy study (Figure 3), the lung parenchyma showed signs of septal fibrosis of irregular distribution with no architectural distortion or remodeling, features of organizing pneumonia, abundant lymphoid follicles (occasionally with a reactive center) and frequent foamy alveolar macrophages. All these results were consistent with APT.
Once the drug was discontinued, there was clinical and radiological improvement of the lung lesions, and the patient was started on corticoid and oxygen therapy.
KL-6 levels were determined on the first day of the patient's hospital stay, 7 days after admission, and 140 days after the drug was withdrawn (695, 1005, and 325 U/L, respectively) using the technique described by Endoh et al.2
DISCUSSION
Amiodarone pulmonary toxicity was first described in 1980 by Rotmensch et al.3 The toxic effects of amiodarone in the lung4 include diffuse alveolar damage, organizing pneumonia, bronchiolitis obliterans, bronchospasm, diffuse hemorrhagic alveolar damage, hypersensitivity pneumonitis, heart failure, pulmonary infiltrates with eosinophilia, and interstitial lung disease. The drug can have a direct mechanism of action through the release of free radicals or an indirect action through hypersensitivity.5 The clinical symptoms vary, but usually include dyspnea, particularly exertional, and dry cough.1 The most frequent radiological alteration is a diffuse interstitial pattern,6 although there are cases of a solitary lung mass4 and less often, pleural involvement or unilateral alveolar infiltrates.4 The literature contains a few cases of APT associated with multiple pulmonary nodules resembling a malignant process,7-9 probably due to an inflammatory response to drug-induced accumulation of phospholipid inclusions in the alveolar cells.10
The pathological findings most frequently include bronchiolitis obliterans, interstitial pneumonitis, interstitial fibrosis, or a combination of all of them.6,11,12 The presence of vacuolated macrophages is frequent, but not pathognomonic of APT since it also appears in patients exposed to the drug who do not develop APT.
Immunohistochemical studies show that KL-6 is strongly expressed in the type 2 pneumocytes regenerated or activated after an interstitial aggression, and therefore could be a good marker of activity in interstitial pulmonary diseases, such as interstitial pneumonia associated with collagen disease, actinic pneumonitis, and amiodarone-induced pneumonitis.2
The diagnosis of APT is costly and difficult on occasions, and is based on the clinical manifestations and radiological findings; and invasive techniques should be used to rule out other processes.
We have presented the first case in Spain of APT documented by histological findings and favorable progress after drug discontinuation, where a significant increase in the marker KL-6 is observed. Although there are still no prospective studies that would help assess its usefulness in the prediction of APT, serum KL-6 is an innovative diagnostic tool for a disease without a clear reference pattern for diagnosis.