The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has revealed several cardiovascular complications, including myocarditis caused by SARS-CoV-2 infection (COVID-19) or after messenger RNA vaccine administration. Because of the high prevalence of COVID-19, the expansion of vaccination programs, and the appearance of new information on myocarditis in these contexts, there is a need to condense the knowledge acquired since the start of the pandemic. To meet this need, this document was drafted by the Myocarditis Working Group of the Heart Failure Association of the Spanish Society of Cardiology, with the collaboration of the Spanish Agency for Medicines and Health Products (AEMPS). The document aims to address the diagnosis and treatment of cases of myocarditis associated with SARS-CoV-2 infection or messenger RNA vaccine administration.
Keywords
Myocarditis is defined as inflammation of the cardiac muscle; its etiology is diverse and includes infectious agents or toxins and autoimmune processes.1 The pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been associated with a series of cardiovascular complications, particularly myocarditis caused both by infection with SARS-CoV-2 itself (COVID-19) and by the administration of messenger RNA (mRNA) vaccines.
The initial studies of patients with COVID-19 showed that 20% to 30% had cardiac troponin levels above the 99th percentile upper reference limit, an indicator of myocardial damage2 that is not always secondary to myocarditis.3–5 Infection with SARS-CoV-2 may cause myocardial damage, in addition to other effects, due to the associated immune response,6–9 a hypercoagulable state with thrombotic events,7 and myocardial ischemia caused by elevated myocardial oxygen demand during the infection (type 2 myocardial infarction). This variety of conditions is reflected in the fact that, in COVID-19 patients with evidence of myocardial damage due to troponin elevation, just 50% of cardiac magnetic resonance (CMR) scans show pathological findings; of these, 27% are compatible with myocarditis, 22% with ischemic heart disease, and the rest are nonspecific.7
With the progression of the pandemic and the vaccination program, evidence has been found of the development of cases of myocarditis after the administration of the mRNA vaccines, particularly in men, adolescents, and young adults. This type of autoimmune mechanism-associated myocarditis is poorly understood and has represented a challenge for the management of vaccination programs.
Given the above, and in light of the accumulated evidence, it is essential to synthesize all of the knowledge acquired in the last 2 years to reach a consensus. Accordingly, the present document, supported by the Myocarditis Working Group of the Heart Failure Association of the Spanish Society of Cardiology and with the collaboration of the Spanish Agency for Medicines and Health Products (AEMPS), addresses the diagnosis and management of cases of myocarditis associated with SARS-CoV-2 infection or mRNA vaccines.
PATHOPHYSIOLOGY AND HISTOPATHOLOGYMyocarditis is pathophysiologically defined as inflammation of the myocardium characterized by inflammatory infiltrates and myocardial damage without an underlying ischemic etiology.10 Its most frequent cause in developed countries is viral.10 Several cases of acute myocarditis in patients with COVID-19 have been published and it is considered an infrequent cardiovascular complication.6,11
The pathophysiological mechanisms of SARS-CoV-2-associated myocarditis are still uncertain and controversial.12 Of the many hypotheses advanced, the most robust is cytotoxicity mediated by a hyperimmune response, in which the excessive release of different inflammatory mediators induces lymphocyte dysregulation that causes T lymphocyte-mediated necrosis.13–15 This hypothesis is in line with most of the reported cases of myocarditis in COVID-19 patients without detection of viral RNA in the myocardium.13,15 The evidence for direct myocardial damage is very weak13,15 because, although SARS-CoV-2 RNA has been found in autopsies16 and endomyocardial biopsies,9 it is localized to interstitial cells and only a minority meets the histopathological criteria of myocarditis.13,15
There are various hypotheses concerning the mechanisms underlying cardiac involvement after vaccination against COVID-19 with mRNA vaccines,17,18 which include a hyperimmune or inflammatory response triggered by mRNA molecules, hypersensitivity to the vaccine or some of its components, and autoimmune disorders mediated by molecular mimicry, but there are insufficient data to confirm or refute any of these theories. A recent study19 demonstrated the presence of antibodies against interleukin-1 receptor antagonist and proposed the transient loss of peripheral immune tolerance as a possible mechanism. From the histological perspective, lymphocytic myocarditis is generally observed and, in very rare cases, eosinophilic myocarditis, as described for other vaccines (rubella, smallpox, and polio).20
EPIDEMIOLOGYEpidemiology of post-COVID-19 myocarditisThe true prevalence of myocarditis in patients with COVID-19 is unknown. The saturation of the health care system in the first few months of the pandemic and the clinical treatment driven by the respiratory symptoms undermined the identification of the more severe cardiac features. In addition, the numerous published reports did not differentiate between myocardial damage and myocarditis and used varying diagnostic approaches.
In patients hospitalized with COVID-19, the average prevalence of definitive/probable myocarditis was estimated to be 2.4 cases/1000 hospitalized patients21 and, in this context, there may have been elevated mortality (20.4%).22 Beyond the confines of the hospital setting, the risk of myocarditis increased by about 10 times in the month after a positive SARS-CoV-2 test, and its development was more frequent in men (60%).23
Echocardiographic changes have been reported to occur in up to 40% of hospitalized patients, although not all of these changes might be secondary to myocarditis.24 Some CMR studies have described findings compatible with myocarditis in hospitalized patients, such as nonischemic late enhancement and native T1 and T2 prolongation in 20%, 73%, and 60%, respectively.25,26 However, in young asymptomatic or paucisymptomatic patients, cardiac involvement is seen in just 0.5% to 3% of cases.27
The autopsy findings of persons with COVID-19 revealed a highly variable cardiac involvement. The largest work identified classic myocarditis in 7.2% of autopsies, with a predominace of other types of changes, such as cellular ischemia, inflammatory infiltration without myocarditis, and microvascular and macrovascular thrombi.28
Epidemiology of postvaccination myocarditisWith the initiation of the vaccination campaign for young people, cases were notified of myocarditis and/or pericarditis after administration of the mRNA vaccines (Comirnaty, BioNTech, Germany/Pfizer, United States; and Spikevax, Moderna, United States).29–33 In contrast to the myocarditis associated with COVID-19, postvaccination myocarditis generally shows a benign course.34
The available data were reviewed by the Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency,35 which concluded that myocarditis and pericarditis are adverse reactions that can occur after mRNA vaccine administration, with a very low frequency overall and with highest risk for young men several days after the second dose. Their generally benign clinical course means that the risk-benefit balance is still favorable after vaccination, considering the efficacy of the vaccines in preventing COVID-19-related hospitalizations and deaths. Similar conclusions were reached by the American Centers for Disease Control and Prevention.36
Two epidemiological studies conducted in France37 and Nordic countries38 confirmed an increased risk of myocarditis and pericarditis after mRNA vaccine vaccination, particularly in young males (12-29 years) in the first week after administration of the second dose and with a frequency depending on the vaccine administered (13 cases with Spikevax and 3 cases with Comirnaty per 100 000 young people vaccinated).
The data on the risk after a third dose are scarce39; the incidence of myocarditis in a cohort of young people aged between 18 and 24 years was estimated to be 11/100 000 vaccinated in the 2 weeks after the third dose,40 and there are no clear differences between the second and third doses.
DIAGNOSISThe diagnosis of myocarditis related to infection with or vaccination against SARS-CoV-2 starts with the presence of symptoms in an epidemiological context of infection or recent administration of an mRNA vaccine. When persistent chest pain, exertional dyspnea or asthenia, palpitations, or syncope develop, the possibility of myocarditis should always be considered, as it can also present as cardiogenic shock or sudden cardiac death.41 The presence of pain with pericardial characteristics is more specific for the frequent joint inflammation of the myocardium and pericardium.
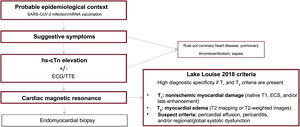
In line with the proposed diagnostic algorithm (figure 1), when myocarditis is clinically suspected, 12-lead electrocardiography (ECG) should always be performed, as well as serial measurement of high-sensitivity cardiac troponin (hs-cTn) and transthoracic echocardiography (TTE). The detection of myocardial damage by hs-cTn concentrations above the 99th percentile upper reference limit is the test best supporting the clinical diagnosis of suspected myocarditis in the absence of coronary heart disease or other causes of myocardial damage. ECG supports this diagnosis if there are compatible alterations, particularly ST-segment elevation. The diagnosis is also supported by the presence of TTE changes, particularly pericardial effusion and/or segmental contractility changes.42
Diagnostic algorithm for myocarditis due to infection with or vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). ECG, electrocardiography; ECS, extracellular space; hs-cTn, high-sensitivity cardiac troponin; mRNA, messenger RNA; TTE, transthoracic echocardiography.
In all cases, confirmation should be made using CMR based on the modified Lake Louise criteria,43 which have elevated sensitivity and specificity in the acute phase for clinical features with considerable clinical suspicion (figure 2).
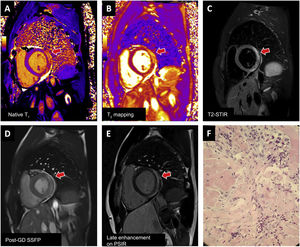
Examples of myocarditis after mRNA vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) confirmed with cardiac magnetic resonance imaging and endomyocardial biopsy. Involvement of the basal lateral face (arrow) meets T1 (A: prolongation of native T1 time; E: late enhancement) and T2 (B: prolongation of T2 time; C: hyperintense signal in T2-weighted images) criteria and is visible in the cine sequence performed after contrast administration (D). Biopsy (F) shows mild lymphoplasmacytic inflammation with eosinophils, interstitial edema, and minimal necrosis of cardiomyocytes. Post-GD SSFP, steady-state free precession after administration of contrast agent (gadolinium); PSIR, phase-sensitive inversion recovery; STIR, short tau inversion recovery.
Endomyocardial biopsy is not necessary for the diagnosis of this form of myocarditis but should be performed if an alternative diagnosis is being considered and, in particular, if there are findings of recurrent sustained ventricular arrhythmias or cardiogenic shock,44,45 to improve therapeutic decision-making.
MANAGEMENTThe management of myocarditis after infection with or vaccination against SARS-CoV-2 is currently a major challenge, given the uncertainty surrounding its pathophysiology and the absence of randomized clinical trials in both contexts. Accordingly, both American41 and European46 consensus documents propose a management similar to that of other viral myocarditides that is mainly based on general supportive treatment with scarce and weak recommendations for specific treatments.
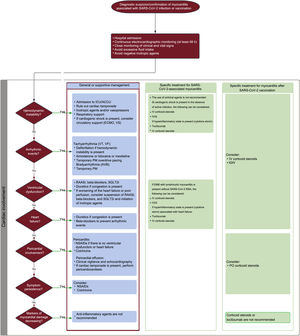
The treatment algorithm can be seen in figure 3. All patients with elevated clinical suspicion of myocarditis due to SARS-CoV-2 infection or vaccination must be admitted for clinical monitoring and ECG for at least 48hours; if possible, the confirmatory study (CMR) should be performed at this time. Admission to a critical care unit is recommended in patients with hemodynamic and/or electrical instability.15,41,46 In general, routine clinical and pharmacological therapy will be applied if arrhythmias, systolic dysfunction, heart failure, and/or pericardial involvement develop. If indicated, beta-blocker and renin-angiotensin-aldosterone system inhibitor regimens should be maintained for at least the first 3 months after hospital discharge.20,46 The use of anti-inflammatory agents is reserved for patients with persistent symptoms and they are generally not necessary for patients with early symptomatic improvement.
Treatment algorithm for myocarditis due to infection with or vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). ACCU, acute cardiac care unit; AVB, atrioventricular block; CVP, central venous pressure; ECMO, extracorporeal membrane oxygenation; EMB, endomyocardial biopsy; ICU, intensive care unit; IVIG, intravenous immunoglobulin; MV, mechanical ventilation; NIMV, noninvasive mechanical ventilation; NSAIDs, nonsteroidal anti-inflammatory drugs; PO, per oral; PM, pacemaker; RAASi, renin-angiotensin-aldosterone system inhibitor; SGLT2i, sodium-glucose cotransporter-2 inhibitor; VF, ventricular fibrillation; VS, ventricular support; VT, ventricular tachycardia.
Regarding the specific treatment of COVID-19-associated myocarditis, some authors indicate a possible benefit of high-dose steroids and intravenous immunoglobulin (IVIG) because the condition can be considered an immune-mediated myocarditis.47 The systematic use of corticosteroids is not generally recommended in myocarditis46 and there is little evidence concerning myocarditis in the context of COVID-19. Nonetheless, based on the benefit shown with dexamethasone in hospitalized patients with COVID-19 and respiratory involvement,48 they are recommended in this type of patient.41,46,48 Regarding IVIG in myocarditis not associated with COVID-19, a meta-analysis49 reported improved survival and ventricular function with its administration with corticosteroids. There is currently no evidence supporting its use in SARS-CoV-2-associated myocarditis.
Other immunomodulatory therapies (tocilizumab, sarilumab, baricitinib, and anakinra) are promising and are currently being studied in relation to SARS-CoV-2-associated myocarditis.41 The positive results for tocilizumab in reducing pulmonary inflammation in COVID-19 patients with cytokine storm50 justify their use in conjunction with systemic steroids in patients with a concomitant hyperinflammatory state and myocarditis.15,41,46,51
PROGNOSIS AND FOLLOW-UPPrognosis based on initial symptomsAlthough numerous cases of clinically suspected myocarditis have been reported in patients with SARS-CoV-2 infection, few cases have been histologically confirmed.9 Initially, due to a selection bias for severe hospitalized patients, fulminant presentations were reported, with elevated need for inotropic or mechanical support. In the context of severe patients with associated pneumonia, mortality can be 6.6% at 3 months.21 Among the patients with fulminant myocarditis, those without associated multisystem inflammatory syndrome (MIS) show a more aggressive cardiological course, with greater need for mechanical support and higher mortality.52 In addition, there is a prognostic implication in the detection of myocardial damage, not only due to the myocarditis, and it has been correlated with an increase in readmissions53 and 30-day mortality in patients admitted for COVID-19.54 The presence of cardiovascular risk factors and/or previous heart disease has been associated with more severe clinical symptoms and worse prognosis, especially in patients with a history of heart disease, whose mortality can be as high as 40%.55–57
The prognosis of the initially more benign clinical symptoms, particularly in the out-of-hospital setting, is less well defined and is limited to series of patients with different inclusion and diagnostic criteria. For example, in a case-control study with young symptomatic outpatients after COVID-19, the prevalence of myocarditis on CMR was 8%, with subsequent progressive improvement in enhancement and without clinical events during follow-up.58
The incidence of myocarditis in the case of reinfection with SARS-CoV-2 is unknown, as well as its characteristics with respect to cases found during the initial infection.
Finally, most cases of myocarditis related to vaccination against SARS-CoV-2 are mild and resolve within 1 to 3 weeks.20 More than 90% of patients have a complete clinical recovery in the first 3 to 6 months.17 Severe cases are infrequent, without differences by sex, and no relationship has been found with previous heart disease.59
Clinical follow-up and imaging testsOutpatient follow-up is recommended after hospital discharge. Clinical evaluation with ECG and TTE is mandatory in all patients, although there are no definitive guidelines on the required intervals. In patients with early improvement without ventricular dysfunction and mild extension in the CMR study, follow-up should be performed at 3 to 6 months. In patients with higher risk (ventricular dysfunction, extensive myocarditis, high arrhythmic risk), early follow-up seems appropriate. In patients with high arrhythmic risk, Holter-ECG is advisable. If clinical status worsens during follow-up, hospitalization, CMR, and/or endomyocardial biopsy should be considered, depending on the severity of the disease.41
Regarding the imaging follow-up of myocarditis of other etiologies, a CMR study at 6 to 12 months of follow-up allows stratification of prognosis according to edema persistence, the absence of a reduction in late enhancement vs the acute-phase study,60 and the localization pattern of residual enhancement.61 In the context of myocarditis due to SARS-CoV-2 infection or vaccination, there are no long-term evidence or established guidelines on the optimal follow-up time for imaging tests. From the available evidence, changes in the native T1 value and late enhancement seem to persist at 3 months vs controls and to disappear at 4 to 6 months after COVID-19-associated myocarditis.62,63 For their part, small series show a normalization of ventricular function and myocardial edema, with persistence of late enhancement 3 months after postvaccination myocarditis,64 which seems to fall until reaching a minimal residual level and without adverse clinical events at 6 months.65 In patients with mild COVID-19 and without initial cardiovascular involvement, CMR does not show changes at 6 months of follow-up.66 Accordingly, taking into account the available data, it seems prudent to propose a follow-up that includes CMR67,68 at about 6 months after diagnosis, which can be brought forward in patients with persistent symptoms who have experienced complications (ventricular arrhythmias or dysfunction), high-level athletes, or at-risk professionals.69
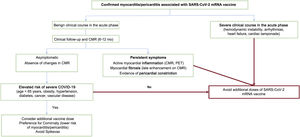
Vaccination after postvaccination myocarditis/pericarditisThere are no solid data on the possible measures that could minimize the risk of myocarditis/pericarditis development. Some results suggest that a gap between the first and second doses exceeding 56 days could decrease the probability of myocarditis/pericarditis onset70 without impacting vaccine efficacy.71 However, these last results are based on pre-Omicron data, which is why their extrapolation to subsequent epidemiological scenarios might be limited.
There are currently no vaccines available in Spain that represent an alternative for patients diagnosed with myocarditis/pericarditis associated with administration of an mRNA vaccine. Adenovirus-based vaccines are not recommended in young people due to the possible development of an infrequent syndrome called severe thrombotic thrombocytopenia. Recently, myocarditis/pericarditis has been identified as a possible adverse reaction to the available protein-based vaccine (Nuvaxovid, Novavax, United States).
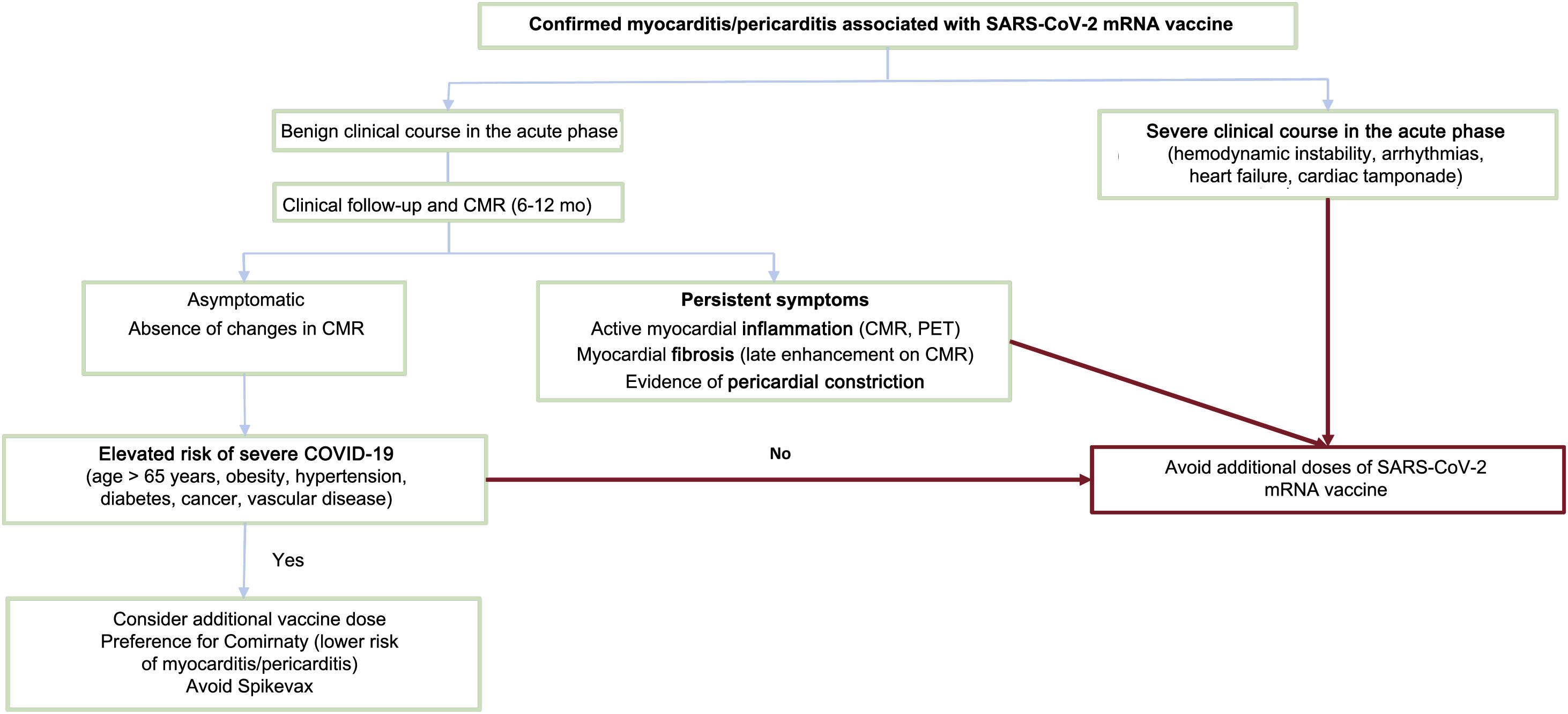
Equally, there is no scientific evidence enabling determination of the optimal approach to patients who have developed myocarditis or pericarditis after administration of a vaccine dose. From the clinical perspective and according to the principles of prudence and biological plausibility, the general recommendation is to not administer additional doses to this group of patients, although it seems reasonable to individualize the intervention based on each patient's characteristics. The proposed algorithm is illustrated in figure 4.
Algorithm for determining the administration of new doses of an mRNA vaccine against SARS-CoV-2 to patients with previous postvaccination myocarditis. CMRI, cardiac magnetic resonance imaging; mRNA, messenger RNA; PET, positron emission tomography; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
The incidence of symptomatic infection with SARS-CoV-2 in pediatric patients is low (1%-2%) and the symptoms are typically mild or nonexistent.72 In addition to respiratory involvement, MIS has been reported in children (MIS-C); it is an infrequent postinflammatory complication (in 8% of hospitalized children; in 0.5%-3% of the general pediatric population with COVID-19) but with a potentially severe clinical course. MIS-C is related to poor immunoregulation in individuals with genetic susceptibility, more than with a direct effect of the virus. Its immunological profile and clinical manifestations differ from those of acute COVID-19 infection67 and share overlapping features with those of other inflammatory diseases.73 MIS-C appears 2 to 6 weeks after the first infection with SARS-CoV-2 and progresses with cardiovascular involvement in most patients; ventricular dysfunction, myocarditis, arrhythmias, and coronary inflammation have been reported72,74–76 and up to 64% of patients may require intensive pediatric care.77 The diagnosis of MIS-C requires evidence of active or previous infection with SARS-CoV-2 and must include ECG, blood tests, and imaging tests, with echocardiography recommended in all patients. In patients with suspected myocardial damage, CMR should be performed in the acute phase and at 1 to 6 months of follow-up in patients with an initial pathological CMR or with coronary aneurysms.67 The management of MIS-C includes respiratory and hemodynamic support in the most severe cases78 and involves drugs in most patients. The first-line immunomodulatory treatment includes IVIG and systemic corticotherapy, although it may require anakinra and tocilizumab.75 In addition, antiplatelet and antithrombotic therapy is used in selected patients. Antiviral agents are not currently recommended. Prognosis is favorable in most patients, although a mortality rate of 1% has been reported in patients with more severe symptoms.78
Vaccination against COVID-19 is approved for individuals aged 5 years or older, and the main scientific societies recommend systematic vaccination of the pediatric population.78,79
AthletesMyocarditis is one of the causes of sudden cardiac death in athletes.80 Accordingly, the initial reports of an elevated incidence of COVID-19-associated myocarditis in athletes raised strong concerns.27 However, more recent registries indicate that cases of myocarditis are rare in athletes who have had COVID-19 (0.4%).81,82
To decrease the risk of severe cardiac events after COVID-19, numerous protocols have been proposed to screen athletes before they resume training or competitive sports. In addition to achieving the safe resumption of sports activity, these protocols must avoid unnecessary limitations, so that athletes can restart their regular physical activity as soon as possible, given the major associated cardiovascular, metabolic, psychological, and emotional benefits.
The screening protocols after COVID-19 infection or vaccination against COVID-19 should be based on the severity of the clinical symptoms and the presence of cardiac symptoms. According to some of the current protocols,41,83 asymptomatic athletes or those with a less than 1-week history of mild noncardiac symptoms (fever, cough, odynophagia, general malaise, myalgia) do not require any tests to return gradually to sports activity as long as they are asymptomatic (excluding anosmia and ageusia) and have completed the required self-isolation. In these cases, and as for other common viruses, medical assessment is not necessary.84 However, athletes who have experienced cardiopulmonary symptoms (chest pain or tightness, palpitations, dyspnea, syncope, and presyncope) or have persistent (> 7 days) symptoms or who have required hospitalization with suspected cardiac involvement must always undergo medical assessment before resuming sports activity.41,83 In these cases, the evaluation should include ECG, echocardiography, and measurement of the hs-cTn and, in if there are pathological findings, confirmation with CMR.
If myocarditis is confirmed after COVID-19 infection or vaccination, moderate- or high-intensity physical exercise is not recommended for 3 to 6 months.85 Resumption of sports activity must be gradual, once the athlete is asymptomatic, with normal functional capacity and without arrhythmias on maximal cardiac stress testing and Holter-ECG (long-duration with a physical exercise session included) and without data on the residual involvement in CMR (preserved left ventricular ejection fraction and absence of edema and/or fibrosis).85 However, we recommend individual assessment of all patients and propose a specific follow-up.
The recommendations collected in the present document are summarized in table 1.
Recommendations for the diagnosis, management, follow-up, and eventual vaccination of patients with SARS-CoV-2 infection- or vaccination-associated myocarditis.
| Diagnosis |
| ECG, hs-cTn, and TTE should be performed in the presence of clinical suspicion of myocarditis and a probable epidemiological context |
| Myocardial damage is defined as a hs-cTn elevation exceeding the 99th percentile upper reference limit, which is the finding that best supports a presumptive diagnosis |
| Alternative diagnoses must be screened (ischemic heart disease, pulmonary thromboembolism, sepsis) |
| Confirmation with CMR is recommended in all patients with clinical suspicion and presence of myocardial damage |
| Endomyocardial biopsy is reserved for patients with suspicion of an alternative diagnosis, ventricular arrhythmias, or hemodynamic instability |
| Treatment |
| Hospitalization and ECG monitoring for at least 48 h are recommended |
| Admission to an acute critical care unit is recommended if there are arrhythmias and/or hemodynamic instability |
| Heart failure should be managed according to standard care |
| Follow-up |
| Clinical follow-up should be performed after hospital discharge and include ECG and TTE |
| Before their reincorporation, CMR is recommended at 6 mo after diagnosis, particularly in patients with persistent symptoms, arrhythmias, or ventricular dysfunction and in high-level athletes and at-risk professionals |
| Vaccination |
| Systematic vaccination is recommended to reduce COVID-19 complications and hospitalizations |
| Myocarditis has been reported to be associated with mRNA vaccines against SARS-CoV-2. They are more frequent in young males and after the second dose |
| In patients who have had postvaccination myocarditis, an additional dose is only recommended in those with a benign clinical course, who remain asymptomatic and without changes in imaging studies, and who have an elevated risk of developing severe COVID-19 |
| Athletes |
| Asymptomatic athletes or those with mild noncardiac symptoms with a duration < 7 d do not require medical assessment or complementary testing to return to sports activity |
| Before resumption of sports activities, athletes with cardiac or persistent (> 7 d) symptoms require a medical assessment including ECG, hs-cTn, and TTE |
| In athletes with confirmed myocarditis, abstention from moderate- and high-intensity physical exercise is recommended for 3 to 6 mo, followed by a gradual reincorporation when symptoms have resolved and there are no pathological findings on complementary examinations in a medical assessment |
CMRI, cardiac magnetic resonance imaging; COVID-19, coronavirus disease 2019; ECG, 12-lead electrocardiography; hs-cTn, high-sensitivity cardiac troponin; mRNA, messenger RNA; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TTE, transthoracic echocardiography.
Myocarditis associated with COVID-19 or occurring after vaccination against SARS-CoV-2 are relatively prevalent entities with a thus far largely unclear pathophysiology. The development of new variants and the administration of vaccine doses require the establishment of shared diagnostic, clinical management, and follow-up guidelines for these patients. The present document reviews the available evidence and proposes algorithms for use in clinical practice.
FUNDINGThe present work has not received funding.
AUTHORS’ CONTRIBUTIONSAll authors participated equally in the drafting of this document.
CONFLICTS OF INTERESTNo conflicts of interest.