Hyperoxygenation after coronary reperfusion causes reperfusion injury, partly as a result of macrophage infiltration contributing to activation of extracellular matrix metalloproteinases (MMP), the main effectors of ventricular necrosis.1 Extracellular-matrix-metalloproteinase-inducer (EMMPRIN) is an essential activation factor.2 A murine coronary ischemia-reperfusion (IR) model has shown the importance of EMMPRIN as a target for the treatment of acute myocardial infarction,1 and more recently, the use of EMMPRIN-targeted magnetic nanoparticles (Figure 1) has been shown to be a potential therapeutic tool for preventing necrosis.3 Before they are studied in a clinical setting, we aimed to evaluate the effectiveness of a porcine coronary IR model.
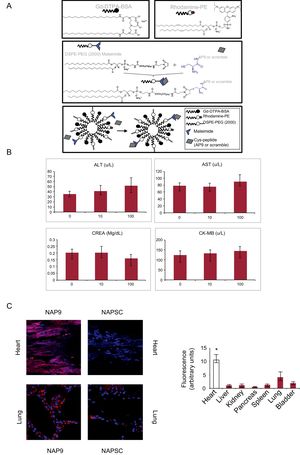
A, Structure of NAP9 or scramble control peptide (NAPSC). B, Production of ALT, AST, creatinine, and CK-MB after administration of 10 mg/kg of NAP9 (n = 5). C, Left panel, detection of NAP9 and NAPSC by confocal microscopy is shown in sections of heart and lung after 7 days of ischemia-coronary reperfusion and injection of 10 mg/kg of NAP9 or NAPSC. Right panel, distribution of NAP9 after 7 days of ischemia-coronary reperfusion and injection of 10 mg/kg of NAP9 in the tissues indicated (n = 10). The plots show mean (SD) values. ALT, alanine transaminase; AST, aspartate transaminase; CK-MB, creatine kinase MB isoenzyme; CREA, creatine kinase; NAP9, nanoparticles containing AP9; NASPC, scramble control peptide.
* P > .05, heart vs lung.
The study included 15 female Yorkshire albino pigs. Five of these were infarct-free. The remaining animals were anesthetized with intravenous administration of propofol 2 mL/kg/h and phentanyl 50 mg/kg/h and submitted to 45minutes of occlusion of the anterior descending artery by balloon inflation. The animals were then injected with 10mg/kg of nanoprobe NAP9 (containing EMMPRIN binding peptide AP9) or NAPSC (containing scramble peptide) as control (Figure 1A).3 Myocardial function was assessed before and 7 days after infarction by echocardiography. Tissue samples were examined for the presence of nanoparticles (confocal microscopy), myocardial integrity (histological staining with hematoxylin-eosin), necrotized area (staining with triphenyl tetrazolium), and EMMPRIN, MMP-9, and MMP-13 as necrosis markers.
Cytotoxicity was studied by injecting NAP9 at 0, 10, 50mg/kg and measuring serum concentrations of aspartate transaminase and alanine transaminase as markers of hepatic injury, creatinine as a renal marker, and creatine kinase MB isoenzyme as a marker of cardiac necrosis. Total absence of cytotoxicity occurred at a dose of 10mg/kg (Figure 1B). Biodistribution was analyzed by confocal microscopy of sections of heart, liver, kidney, pancreas, spleen, lung, bladder, and intestine after 7 days of IR (IR7); the heart and lung were the tissues with highest NAP9 uptake (Figure 1C).
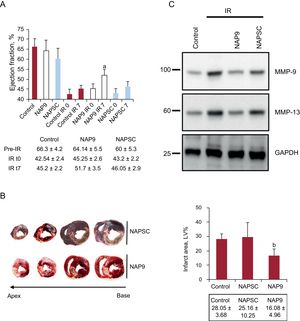
The effect of NAP9 on progression of acute myocardial infarction was studied by injecting 10mg/kg of NAP9 or NPASC after 15minutes of reperfusion of the anterior descending artery. It was found that left ventricular ejection fraction (estimated in B mode by the Simpson biplane method and in M mode [Teichholz method], with similar results) of the pigs injected with NAP9 was significantly greater than in control animals (NAP9 IR7 vs control IR7, 51.7% [3.5%] vs 45.2% [2.2%]; P<.05) (Figure 2A). In contrast, the extent of the necrotized area, expressed as a percentage of the total surface of the left ventricle (control vs NAP9, 28.05% [3.68%] vs 16.08% [4.96%]; P<.0003) (Figure 2B) and expression of MMP-9 and MMP-13 (Figure 2C), indicative of necrosis due to extracellular matrix degradation, decreased in pigs treated with NAP9.
A, The upper panel shows left ventricular ejection fraction (mean [SD] of pigs without infarction or infarcted and injected or not injected with 10 mg/kg of NAP9 or NAPSC (n = 15). The lower panel shows the values of ejection fraction prior to the procedure, after ischemia-reperfusion (IR0) and after 7 days of IR (IR7). B, The left panel shows serial 0.5 cm sections of the hearts of the pigs injected with NAP9 or NAPSC microprobes. The right panel shows the values (mean [SD]) of the infarcted area expressed as percentage of the total ventricular volume (n = 15). C, Detection of metalloproteinases MMP-9 and MMP-13 in the hearts of pigs injected with NAP9 or NAPSC after 7 days of IR. GAPDH was used as control (n = 15). GAPDH, glyceraldehyde 3-phospodehydrogenase; IR, ischemia/reperfusion; MMP, metalloproteinases; NAP9, AP9 peptide; NASC, scramble peptide; LV, left ventricle.
a NAP9 IR7 vs control IR7, P < .05.
b NAP9 vs control, P < .0003.
Nanotechnology applied to the treatment and prevention of reperfusion injury is an approach with promising clinical results.4,5 In conclusion, the extension of infarction was significantly reduced in the pigs that underwent coronary IR and received NAP9, and therefore ventricular function was at least improved through reduction of necrosis associated with degradation of the extracellular matrix. Before studying the approach in clinical trials, and bearing in mind the limitation of using echocardiography compared with magnetic resonance imaging for functional assessment of contractility, it would be necessary to increase the sample size of the study and, depending on the visibility of NAP9 in magnetic resonance imaging, complement the results by noninvasive means with magnetic resonance imaging. This would enable molecular imaging to be used in the future as a tool to assess the utility of EMMPRIN in the treatment of acute myocardial infarction.
FUNDINGThis study received a grant from the Spanish Society of Cardiology project in 2016.


![A, The upper panel shows left ventricular ejection fraction (mean [SD] of pigs without infarction or infarcted and injected or not injected with 10 mg/kg of NAP9 or NAPSC (n = 15). The lower panel shows the values of ejection fraction prior to the procedure, after ischemia-reperfusion (IR0) and after 7 days of IR (IR7). B, The left panel shows serial 0.5 cm sections of the hearts of the pigs injected with NAP9 or NAPSC microprobes. The right panel shows the values (mean [SD]) of the infarcted area expressed as percentage of the total ventricular volume (n = 15). C, Detection of metalloproteinases MMP-9 and MMP-13 in the hearts of pigs injected with NAP9 or NAPSC after 7 days of IR. GAPDH was used as control (n = 15). GAPDH, glyceraldehyde 3-phospodehydrogenase; IR, ischemia/reperfusion; MMP, metalloproteinases; NAP9, AP9 peptide; NASC, scramble peptide; LV, left ventricle. a NAP9 IR7 vs control IR7, P < .05. b NAP9 vs control, P < .0003. A, The upper panel shows left ventricular ejection fraction (mean [SD] of pigs without infarction or infarcted and injected or not injected with 10 mg/kg of NAP9 or NAPSC (n = 15). The lower panel shows the values of ejection fraction prior to the procedure, after ischemia-reperfusion (IR0) and after 7 days of IR (IR7). B, The left panel shows serial 0.5 cm sections of the hearts of the pigs injected with NAP9 or NAPSC microprobes. The right panel shows the values (mean [SD]) of the infarcted area expressed as percentage of the total ventricular volume (n = 15). C, Detection of metalloproteinases MMP-9 and MMP-13 in the hearts of pigs injected with NAP9 or NAPSC after 7 days of IR. GAPDH was used as control (n = 15). GAPDH, glyceraldehyde 3-phospodehydrogenase; IR, ischemia/reperfusion; MMP, metalloproteinases; NAP9, AP9 peptide; NASC, scramble peptide; LV, left ventricle. a NAP9 IR7 vs control IR7, P < .05. b NAP9 vs control, P < .0003.](https://static.elsevier.es/multimedia/18855857/0000007200000002/v2_201904110621/S1885585718301051/v2_201904110621/en/main.assets/thumbnail/gr2.jpeg?xkr=eyJpdiI6IlRhamx4em85a283MFZxWEkxbkRxK0E9PSIsInZhbHVlIjoieWczOU1PZzExbm1oUVJlVzRvZjBuWm5wZDBYMUU3bnIvUjA1dFYxcFZNVT0iLCJtYWMiOiJlMTY2YTFmNDlhZjE2N2RkYzgwYjg5YmU2NDg4NjMzNjIyMmY3Mzc3ZjhkNjljNDI3NjgyOGYzMDg1ZTBhNTMyIiwidGFnIjoiIn0=)