A man aged 49 years, ex-smoker with a family history of premature ischemic heart disease, attended the emergency department after 1 month with clinical symptoms of progressive angina following even minimal exertion. In 2001, he had presented non-Q wave acute myocardial infarction and undergone conventional bare-metal stent (BMS) implantation in the mid left anterior descending artery. In 2007, a coronary angiogram for exertional angina showed no stent restenosis. Following a change in treatment, he remained asymptomatic and recorded negative exercise test results until this admission in 2012.
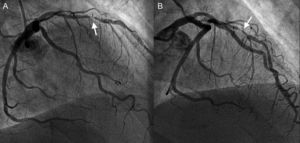
In view of symptoms of unstable angina, a fresh coronary angiography was requested and this showed substantial, focal intrastent restenosis (Fig. 1). An angioplasty guidewire was advanced through the stenosis without complications and a decision was made to use optical coherence tomography for the assessment. The optical coherence tomography showed correct expansion of the stent, absence of uncovered struts, and presence of intrastent neoatherosclerosis (Fig. 2) with lipid-laden intima, a cavity resulting from the rupture of the fibrous cap of a fine-cap fibroatheroma, and an apparently more fibrous proximal portion with images compatible with cholesterol crystal deposits. The intrastent restenosis was predilated with a delivery balloon and a drug-eluting stent implanted.
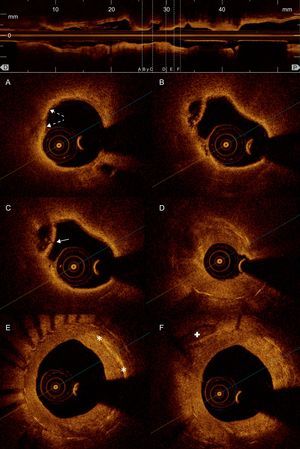
Optical coherence tomography: intrastent restenosis. A: Lipid-laden neointima (low intensity tissue, badly defined borders, and high attenuation that causes a shadow limiting visualization of the stent struts); presents points, superficial, hyperintense areas compatible with macrophage infiltration (broken arrows). B: Cavity caused by the rupture of a neointimal plaque with the remains of its fibrous capsule. C: Ruptured fibrous cap of the same plaque (arrow). D: Heterogeneous neointimal proliferation causing substantial arterial lumen stenosis. E: Very hyperintense linear areas compatible with cholesterol deposits (asterisks) in the neointima. F: Diffuse neointimal and more homogeneous proliferation indicating greater fibrous content, with calcium plaque (cross) outside of the stent.
Bare-metal stent restenosis has traditionally been considered stable and benign, presenting neointimal growth in the first 6 to 12 months followed by a later quiescent period. However, the angiographic clinical course of our patient and optical coherence tomography images support the recent theory that neoatherosclerosis is an active causal mechanism in many cases of restenosis and late stent thrombosis.1 Moreover, symptoms of progressive angina and the discovery of ruptured plaque intrastent confirm that presentation is not consistently in the form of silent ischemia or stable angina and it has been estimated that≤9.5% of BMS restenoses could present as acute coronary syndrome.2
Histopathologic studies have shown that, while neoatherosclerosis is a common process in BMS and drug-eluting stent, its occurrence is neither homogeneous nor simultaneous.3 In drug-eluting stent, incidence is more frequent and it appears early; in BMS, it is quite exceptional at<2 years.3,4 Nonetheless, at-risk lesions (fine-cap fibroatheroma and ruptured plaque) are more frequently detected in BMS restenosis, even though—as in our patient (Figs. 2A-C)—they mostly appear at>5 years post-implantation.3–5
Very little data exist on optical coherence tomography evaluation of late restenosis in BMS. Takano et al.4 studied initial behavior (<6 months) and course (>5 years) of coronary segments with BMS revascularization and found homogeneous neointimal coating in the first phase, but only in the second phase established the presence of calcium deposits, lipid nuclei, or cholesterol crystals accompanied by significant reduction of lumen. Habara et al.5 compared findings on early (<1 year) and late (>5 years) BMS restenosis and described significant differences in neointimal structure, which was more heterogeneous in appearance and much like typical atherosclerotic plaque. Finally, Yonetsu et al.6 also reported greater attenuation and lipid content in late (>48 months) BMS restenosis neointima by comparison with early BMS restenoses.
Given known morbidity and mortality associated with restenosis and stent thrombosis, it is essential we study the pathologic mechanism in greater depth. The rupture and exposure to the circulating blood of prothrombotic lipid material contained in the neointima may be the cause of many thromboses. The present case shows how optical coherence tomography can contribute to our understanding of these mechanisms.
FundingDr Juan Ruiz-García would like to thank the Spanish Society of Cardiology for financial support received through the Hemodynamics and Interventional Cardiology section's 2011 Post-Residency Research Training Grant.
.