We have read with great interest, and no little admiration, the editorial by A.M. Richards “Plasma neprilysin concentrations: a new prognostic marker in heart failure?”,1 which addresses our article “Multimarker strategy for heart failure prognostication. Value of neurohormonal biomarkers: Neprilysin vs NT-proBNP”2 published in the same issue of the journal. We would like to reply to his comments.
We fully agree with the author1 that it remains unknown whether there is a systematic change in plasma neprilysin concentrations in heart failure (HF) compared with the normal state of health. As far as we know, there are no studies on the biological variability of neprilysin that compare concentrations in healthy people and HF patients, and so this should be a key step in increasing our knowledge of this biomarker.
The author states in his editorial that Vodovar et al3 recently reported higher plasma neprilysin concentrations in patients with chronic HF than in those with acute decompensated HF, whereas N-terminal pro-B-type natriuretic peptide (NT-proBNP) concentrations were at their most elevated in decompensated HF. In contrast, we found that neprilysin concentrations were higher in patients with acute HF than in those with chronic HF and that the concentrations tended to decrease with the treatment administered during hospital admission.4
It should be noted that that the authors3 found no correlation between the soluble neprilysin concentrations measured and neprilysin activity. Again, conflicting results have been obtained. In a small series of 98 patients, we found a low but significant correlation (ρ=0.50, P<.001) between soluble neprilysin concentrations and neprilysin activity, which suggests that soluble neprilysin retains some of its catalytic activity.5
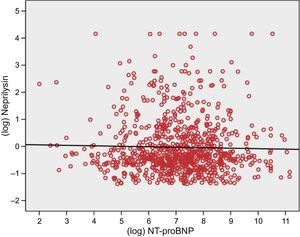
In a direct reference to our article,2 the author suggests that the lack of correlation between neprilysin and NT-proBNP may due to 12% of values appearing below the detection limit, which would confer a flat or “squashed” distribution on a part of the study population. We eliminated all these patients from the analysis and again documented the lack of correlation between neprilysin and NT-proBNP (ρ=-0.02, P=.68, log-transformed r=-0.3, P=.47; Figure). We agree with the author1 that it is hard to understand this finding or its clinical significance.
Finally, we would like to respond to the author's question regarding the noninclusion of neprilysin and NT-proBNP together in the same model, “Are the data presented like this because both markers” fall out “of the model when both are included?”1 The direct answer is no. Firstly, in the article published in Revista Española de Cardiología, our intention was to conduct a head-to-head comparison of neprilysin and NT-proBNP within a multibiomarker strategy, rather than to demonstrate the contribution of neprilysin and NT-proBNP in combination. Secondly, the model that included both biomarkers had been published in our first article on neprilysin,6 in which neprilysin improved reclassification and the hazard ratio of the primary endpoint (cardiovascular death or HF hospitalization) and cardiovascular death when added to a model that already included NT-proBNP.6 We also stated that when the biomarkers ST2 and high-sensitivity troponin T were added to the multibiomarker strategy, neprilysin remained significantly associated with the composite endpoint (hazard ratio=1.15; 95% confidence interval, 1.03-1.28; P=.02) and with cardiovascular death (hazard ratio=1.17, confidence interval 95%, 1.03-1.32; P=.02) in combination with ST2 and troponin T. We also found improvements in the hazard ratio of both endpoints (P=.02 and P=.04, respectively) within the multibiomarker strategy.6 In contrast, NT-proBNP did not do this.
CONFLICTS OF INTERESTJ. Lupón and A. Bayes-Genis have applied for an international patent for the use of soluble neprilysin as a prognostic marker in patients with HF.