Prior studies have not determined whether the effect of dual antiplatelet therapy (DAPT) cessation on the subsequent risk of major adverse cardiac events (MACE) varies by the choice of P2Y12-inhibitor after acute coronary syndrome (ACS).
MethodsWe performed a prespecified subanalysis of a multicenter, prospective registry of ACS patients discharged on ticagrelor or clopidogrel between 2015 and2019. Nonadherence to DAPT was categorized as physician-guided discontinuation and disruption due to adverse effects, nonadherence, or bleeding. The association between DAPT cessation and 1-year MACE was analyzed using multivariate time-updated Cox models with inverse probability of censoring weighted estimators.
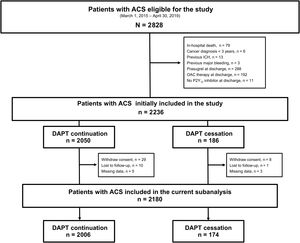
ResultsOut of 2180 patients, 174 (8.3%) prematurely discontinued DAPT (physician-guided, n=126; disruption, n=48). Nonadherent patients were older and had more comorbidities than those on DAPT. Compared with physician-guided discontinuation, disruption occurred earlier after discharge and was more frequent with ticagrelor than with clopidogrel. In time-varying analysis, DAPT cessation was associated with an increased risk of MACE (adjusted HR, 1.32, 95%CI, 1.10-1.76), largely driven by disruption (adjusted HR, 1.47, 95%CI, 1.22-1.73). There was an exponential increase in MACE risk after DAPT cessation within 90 days after ACS, especially after disruption of ticagrelor compared with clopidogrel (Pinteraction<.001). After adjustment for DAPT duration, this interaction was not statistically significant on the additive scale (relative excess risk due to interaction 0.12, 95%CI,−0.99-1.24).
ConclusionsIn this all-comers registry, 1 in 12 patients prematurely discontinued DAPT within 1 year after ACS. Compared with physician-recommended discontinuation, disruption resulted in a significantly higher risk of MACE. After adjustment for DAPT duration, this association was not moderated by the choice of P2Y12-inhibitor.
Clinical trial registered at ClinicalTrials.gov (Identifier: NCT02500290)
Keywords
Current guidelines recommend dual antiplatelet therapy (DAPT) with potent P2Y12 receptor inhibitors (P2Y12-i) over clopidogrel for at least 12 months after acute coronary syndrome (ACS).1 However, despite the widespread implementation of secondary prevention strategies over the last 2 decades, patients with ACS remain at high risk of recurrent ischemic events particularly during the first year after the index event.2–4 Nonadherence to DAPT, especially disruption due to nonadherence or bleeding, is associated with an increased risk of thrombotic events.5–7 Notwithstanding the greater benefit that may derive from potent P2Y12-i, premature ticagrelor discontinuation has been reported in up to 25% of patients in randomized clinical trials (RCTs).4 In this context, compared with clopidogrel, ticagrelor has relatively common adverse effects such as bleeding and dyspnea that may warrant premature disruption of therapy or switching to a less potent agent.5,8–11 Nevertheless, whether the clinical impact of unplanned cessation of DAPT after ACS varies according to the P2Y12-i class remains unknown.11,12 Against these uncertainties, we sought to describe the frequency, determinants, and clinical significance of different nonadherence patterns to DAPT with ticagrelor vs clopidogrel in a contemporary cohort of ACS patients.
METHODSStudy design and populationThis is a prespecified subanalysis (ClinicalTrials.govNCT04630288, Safety and Efficacy of Ticagrelor vs Clopidogrel in Patients With Acute Coronary Syndrome) of the CREA-ARIAM registry (ClinicalTrials.gov Identifier NCT02500290, Antiplatelet Therapy in Acute Coronary Syndrome (ACS). Safety and Efficacy of Switching Antiplatelet), a prospective, multicenter investigator-initiated branch of the ARIAM-Andalucía registry (Analysis of Delay in Acute Myocardial Infarction in Andalucía). Details of design, definitions, and primary results of the ARIAM-Andalucía registry and CREA-ARIAM registry have already been reported elsewhere.13,14 The quality control of data in ARIAM-Andalucía registry is carried out at regular intervals through independent external audits by the Agency for Healthcare Quality of Andalucía. Patients with ACS admitted to cardiac care units between March 2015 and April 2019, were prospectively screened for eligibility if they were intended to receive at least 12 months of DAPT with clopidogrel or ticagrelor. Major exclusions were a history of previous intracranial hemorrhage or recent major bleeding, patients discharged on prasugrel or oral anticoagulation, and patients lost to follow-up or with missing data (methods 1 of the supplementary data). The study was approved by the Regional Research Ethics Committee of Andalusia and the Institutional Review Boards at each participating center. All eligible patients were required to give written informed consent.
Study endpoints and definitionsThe primary endpoint was the first occurrence of major adverse cardiac events (MACE), a composite of all-cause mortality, myocardial infarction,15 stroke, unplanned target lesion revascularization, or definite stent thrombosis at 1 year.16 Secondary endpoints included each individual component of the primary endpoint, and a more restrictive definition of MACE (MACE-2) including cardiovascular death, myocardial infarction, unplanned target lesion revascularization, and definite stent thrombosis. Major bleeding was defined as Bleeding Academic Research Consortium type 3 or 5 bleeding17 (methods 2 of the supplementary data).
Exposure and outcome ascertainmentClinical endpoints and DAPT exposure status were systematically and prospectively tracked throughout 1 year after discharge during scheduled postdischarge outpatient follow-up visits, and by trained research coordinators through structured telephone interviews with patients or relatives planned at 1, 6 and 12 months after discharge (methods 2 of the supplementary data). These included a dedicated questionnaire in which patients were asked to provide detailed information on hospital readmissions, outpatient visits, drug-related adverse effects, or any change in antiplatelet therapy from the last contact. Specifically, we collected dates of stopping and restarting medication, reasons for drug cessation, and the replacement therapy, when applicable. Self-reported information from patients was systematically validated with data manually extracted from electronic medical records. All potential endpoints identified during the follow-up period underwent formal adjudication by consensus between 2 experienced investigators who were blinded to calendar year and DAPT status, using previously anonymized original source data. Additionally, medication adherence was measured at regular intervals irrespective of clinical status by means of the medication possession ratio (methods 2 of the supplementary data). For this study, the term cessation refers to any unplanned DAPT discontinuation before 12 months, defined as temporary interruption (< 14 days), or permanent discontinuation of ticagrelor or clopidogrel for more than 3 days, with or without aspirin cessation. In keeping with the PARIS registry,5 we considered 2 modes of cessation: physician-guided discontinuation and disruption due to nonadherence, adverse effects, or bleeding. Treatment interruption due to surgery or the need for invasive procedures was categorized as physician-recommended discontinuation. We further categorized nonadherence according to the timing of drug discontinuation in relation to hospital discharge into early (< 90 days) and late (>90 days) cessation, and by the P2Y12-i choice at the time of cessation. Additionally, specific reasons for discontinuation were systematically captured and categorized according to the predefined cessation modes.
Statistical analysisBaseline and procedural characteristics according to DAPT cessation status are described using frequencies and percentages for categorical variables, and mean±standard deviation, or median [interquartile range] for continuous variables, as appropriate. Patient characteristics of patients in each cessation group were compared using chi-square tests for categorical variables, and independent samples Student t tests, Mann-Whitney U-tests, or Kruskal-Wallis tests for continuous variables, as appropriate. The inverse probability of censoring weighting (IPCW) approach was used to account for dependent censoring resulting from time-varying confounders18 (methods 2 of the supplementary data). The cumulative incidence of different nonadherence patterns was summarized using weighted Kaplan-Meier estimators. Predictors of premature cessation were assessed using multivariate time-updated Cox regression models using the multivariate fractional polynomials approach.19 The adjusted risk of MACE associated with DAPT cessation was analyzed taking death as a competing event, fitting multivariate time-updated Cox regression models with each cessation mode entered as a time-varying covariate. Models included doubly robust IPCW estimators with participating hospitals entered as cluster random-effect variable using robust estimators to account for the lack of independence induced by the weighted data, and interhospital variability in clinical performance (methods 2 of the supplementary data). Results are expressed as adjusted hazard ratios (aHR) with 95% confidence intervals (95%CI). We performed subgroup analysis across nonadherence categories stratified by P2Y12-i status using the formal (Wald) test for interactions with uninterrupted DAPT as the reference group. In addition, interactions were assessed on the additive scale using the relative excess risk due to interaction (RERI) and the attributable proportion, with 95%CI calculations by the delta method.20 All subgroup analyses were deemed exploratory and no adjustments for multiplicity were applied. To ensure the robustness of the main results, a set of sensitivity analyses were performed: a) for different datasets to address reverse causality, excluding patients more likely to discontinue DAPT; b) fitting flexible parametric survival models using restricted cubic spline functions to model DAPT duration as a continuous covariate; and c) according to PARIS criteria, considering DAPT interruption due to surgery separately. All analysis tests were 2-tailed with alpha set at 5% and performed using Stata 14.2 (Stata Corp 2016. Stata Statistical Software: College Station, Stata Corp LP, United States).
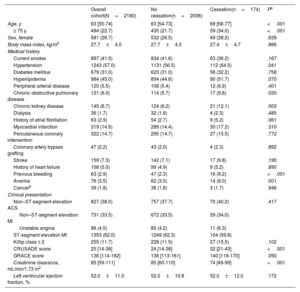
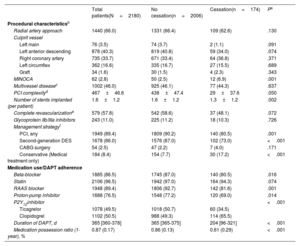
RESULTSPatient characteristicsOut of 2828 patients screened, 2180 were included in this analysis. Of these, 174 (8.3%) patients discontinued DAPT, 126 (6.0%) were categorized as physician-guided discontinuation, and 48 (2.4%) as disruption (figure 1). The annual rate of DAPT cessation significantly decreased over the study period (Pfor trend=.035) (Figure 1 of the supplementary data). Table 1 and table 2 summarize patient demographics, clinical and procedural characteristics, and medication use according to DAPT cessation status. Compared with patients on DAPT, those who prematurely discontinued treatment were older, more frequently had prior bleeding, impaired renal function, chronic obstructive pulmonary disease or anemia, and were more likely to have myocardial infarction with nonobstructive coronary arteries and receive medical treatment only for the index ACS. In contrast, they were less likely to receive drug-eluting stents and guideline-directed medical therapies at hospital discharge, including potent P2Y12-i. There were no between-group differences in clinical presentation upon admission, the prevalence of multivessel disease, or completeness of revascularization, nor were there major differences when we analyzed modes of cessation (tables 1-2 of the supplementary data).
Baseline characteristics of patients according to dual antiplatelet therapy cessation status
| Overall cohort(N=2180) | No cessation(n=2006) | Cessation(n=174) | Pa | |
|---|---|---|---|---|
| Age, y | 63 [55-74] | 63 [54-73] | 69 [58-77] | <.001 |
| ≥ 75 y | 494 (22.7) | 435 (21.7) | 59 (34.0) | <.001 |
| Sex, female | 581 (26.7) | 532 (26.5) | 49 (28.2) | .639 |
| Body mass index, kg/m2 | 27.7±4.0 | 27.7±4.3 | 27.4±4.7 | .866 |
| Medical history | ||||
| Current smoker | 897 (41.0) | 834 (41.6) | 63 (36.2) | .167 |
| Hypertension | 1243 (57.0) | 1131 (56.5) | 112 (64.5) | .041 |
| Diabetes mellitus | 679 (31.0) | 623 (31.0) | 56 (32.2) | .758 |
| Hyperlipidemia | 984 (45.0) | 894 (44.6) | 90 (51.7) | .070 |
| Peripheral arterial disease | 120 (5.5) | 108 (5.4) | 12 (6.9) | .401 |
| Chronic obstructive pulmonary disease | 131 (6.0) | 114 (5.7) | 17 (9.8) | .030 |
| Chronic kidney disease | 145 (6.7) | 124 (6.2) | 21 (12.1) | .003 |
| Dialysis | 36 (1.7) | 32 (1.6) | 4 (2.3) | .485 |
| History of atrial fibrillation | 63 (2.9) | 54 (2.7) | 9 (5.2) | .061 |
| Myocardial infarction | 319 (14.6) | 289 (14.4) | 30 (17.2) | .310 |
| Percutaneous coronary intervention | 322 (14.7) | 295 (14.7) | 27 (15.5) | .772 |
| Coronary artery bypass grafting | 47 (2.2) | 43 (2.0) | 4 (2.3) | .892 |
| Stroke | 159 (7.3) | 142 (7.1) | 17 (9.8) | .190 |
| History of heart failure | 108 (5.0) | 99 (4.9) | 9 (5.2) | .890 |
| Previous bleeding | 63 (2.9) | 47 (2.3) | 16 (9.2) | <.001 |
| Anemia | 76 (3.5) | 62 (3.0) | 14 (8.0) | .001 |
| Cancerb | 39 (1.8) | 36 (1.8) | 3 (1.7) | .946 |
| Clinical presentation | ||||
| Non–ST-segment elevation ACS | 827 (38.0) | 757 (37.7) | 70 (40.2) | .417 |
| Non–ST-segment elevation MI | 731 (33.5) | 672 (33.5) | 59 (34.0) | |
| Unstable angina | 96 (4.5) | 85 (4.2) | 11 (6.3) | |
| ST-segment elevation MI | 1353 (62.0) | 1249 (62.3) | 104 (59.8) | |
| Killip class ≥ 2 | 255 (11.7) | 228 (11.5) | 27 (15.5) | .102 |
| CRUSADE score | 25 [14-38] | 24 [14-38] | 32 [21-43] | <.001 |
| GRACE score | 136 [114-162] | 136 [113-161] | 140 [118-170] | .050 |
| Creatinine clearance, mL/min/1.73 m2 | 85 [59-111] | 85 [60-110] | 74 [49-99] | <.001 |
| Left ventricular ejection fraction, % | 52.0±11.0 | 52.0±10.8 | 52.0±12.0 | .172 |
ACS, acute coronary syndrome; CRUSADE, Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA guidelines; GRACE, Global Registry of Acute Coronary Evets; MI, myocardial infarction.
Data are expressed as No. (%), mean±SD, or median [interquartile range].
Procedural characteristics, medication use and adherence by DAPT cessation status
| Total patients(N=2180) | No cessation(n=2006) | Cessation(n=174) | Pa | |
|---|---|---|---|---|
| Procedural characteristicsb | ||||
| Radial artery approach | 1440 (66.0) | 1331 (66.4) | 109 (62.6) | .130 |
| Culprit vessel | ||||
| Left main | 76 (3.5) | 74 (3.7) | 2 (1.1) | .091 |
| Left anterior descending | 878 (40.3) | 819 (40.8) | 59 (34.0) | .074 |
| Right coronary artery | 735 (33.7) | 671 (33.4) | 64 (36.8) | .371 |
| Left circumflex | 362 (16.6) | 335 (16.7) | 27 (15.5) | .689 |
| Graft | 34 (1.6) | 30 (1.5) | 4 (2.3) | .343 |
| MINOCA | 62 (2.8) | 50 (2.5) | 12 (6.9) | .001 |
| Multivessel diseasec | 1002 (46.0) | 925 (46.1) | 77 (44.3) | .637 |
| PCI complexityd | 467±46.6 | 438±47.4 | 29±37.6 | .050 |
| Number of stents implanted (per patient) | 1.6±1.2 | 1.6±1.2 | 1.3±1.2 | .002 |
| Complete revascularizatione | 579 (57.8) | 542 (58.6) | 37 (48.1) | .072 |
| Glycoprotein IIb/IIIa inhibitors | 243 (11.0) | 225 (11.2) | 18 (10.3) | .726 |
| Management strategyf | ||||
| PCI, any | 1949 (89.4) | 1809 (90.2) | 140 (80.5) | .001 |
| Second-generation DES | 1678 (86.0) | 1576 (87.0) | 102 (73.0) | <.001 |
| CABG surgery | 54 (2.5) | 47 (2.2) | 7 (4.0) | .171 |
| Conservative (Medical treatment only) | 184 (8.4) | 154 (7.7) | 30 (17.2) | <.001 |
| Medication use/DAPT adherence | ||||
| Beta-blocker | 1885 (86.5) | 1745 (87.0) | 140 (80.5) | .016 |
| Statin | 2106 (96.5) | 1942 (97.0) | 164 (94.3) | .074 |
| RAAS blocker | 1948 (89.4) | 1806 (92.7) | 142 (81.6) | .001 |
| Proton-pump inhibitor | 1688 (76.5) | 1548 (77.2) | 120 (69.0) | .014 |
| P2Y12inhibitor | <.001 | |||
| Ticagrelor | 1078 (49.5) | 1018 (50.7) | 60 (34.5) | |
| Clopidogrel | 1102 (50.5) | 988 (49.3) | 114 (65.5) | |
| Duration of DAPT, d | 365 [360-378] | 365 [365-375] | 204 [96-321] | <.001 |
| Medication possession ratio (1-year), % | 0.87 (0.17) | 0.86 (0.13) | 0.61 (0.29) | <.001 |
ACS, acute coronary syndrome; CABG, coronary artery bypass grafting; DAPT, dual antiplatelet therapy; DES, drug-eluting stent; MINOCA; myocardial infarction with nonobstructive coronary arteries; PCI, percutaneous coronary intervention; RAAS, renin-angiotensin-aldosterone system.
Data are summarized as No. (%), mean±standard deviation, and median [interquartile range], as appropriate.
Multivessel disease defined as at least 2 major vessels (≥ 2mm diameter) from a different territory with lesions deemed angiographically significant (≥ 50% stenosis of the left main stem, ≥ 70% stenosis in other major coronary vessel, or 30% to 70% stenosis with fractional flow reserve ≤ 0.8)
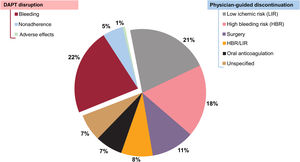
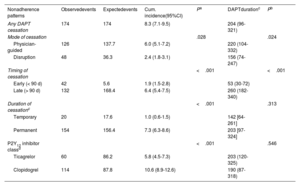
Table 3 depicts the cumulative incidence and timing of DAPT cessation according to the predefined nonadherence categories. Out of 174 episodes of unplanned cessation, most (88.5%) were categorized as permanent, whereas temporary interruptions occurred in a minority for a median of 5.5 [5-9.5] days. Most discontinuations (90%) were for P2Y12-i only, while a minority included both aspirin and P2Y12-i. In the overall cohort, DAPT cessation was most commonly driven by physician decision (figure 2). The median time until any DAPT cessation occurrence was 204 [96-321] days. Compared with physician-guided discontinuation, DAPT disruption, in particular that due to nonadherence, resulted in shorter courses of treatment (table 3 of the supplementary data). The cumulative incidence of cessation for clopidogrel doubled that of ticagrelor, mainly driven by more frequent physician-guided discontinuation (table 3, figure 2 of the supplementary data). By contrast, disruption rates were proportionally higher in ticagrelor-treated patients than in those with clopidogrel, even though, differences were only evident within 90 days after discharge (table 4 of the supplementary data). Further considering the reasons for cessation, differences between ticagrelor- and clopidogrel-treated patients were largely driven by the higher observed rates of noncompliant disruption in the former (figure 3 of the supplementary data). Overall, the duration of DAPT did not differ significantly by type of P2Y12-I (table 3), or the mode and timing of cessation (table 4 of the supplementary data). However, the median time to DAPT disruption within the first 90 days after discharge was significantly shorter with ticagrelor than with clopidogrel (P=.035) (figure 4 of the supplementary data).
Cumulative incidence and timing of dual antiplatelet therapy cessation according to the predefined patterns of nonadherence
| Nonadherence patterns | Observedevents | Expectedevents | Cum. incidence(95%CI) | Pa | DAPTdurationc | Pb |
|---|---|---|---|---|---|---|
| Any DAPT cessation | 174 | 174 | 8.3 (7.1-9.5) | 204 (96-321) | ||
| Mode of cessation | .028 | .024 | ||||
| Physician-guided | 126 | 137.7 | 6.0 (5.1-7.2) | 220 (104-332) | ||
| Disruption | 48 | 36.3 | 2.4 (1.8-3.1) | 156 (74-247) | ||
| Timing of cessation | <.001 | <.001 | ||||
| Early (< 90 d) | 42 | 5.6 | 1.9 (1.5-2.8) | 53 (30-72) | ||
| Late (> 90 d) | 132 | 168.4 | 6.4 (5.4-7.5) | 260 (182-340) | ||
| Duration of cessationc | <.001 | .313 | ||||
| Temporary | 20 | 17.6 | 1.0 (0.6-1.5) | 142 [64-261] | ||
| Permanent | 154 | 156.4 | 7.3 (6.3-8.6) | 203 [97-324] | ||
| P2Y12 inhibitor classd | <.001 | .546 | ||||
| Ticagrelor | 60 | 86.2 | 5.8 (4.5-7.3) | 203 (120-325) | ||
| Clopidogrel | 114 | 87.8 | 10.6 (8.9-12.6) | 190 (87-318) |
95%CI, 95% confidence interval; Cum., cumulative; DAPT, dual antiplatelet therapy.
Data are the number of observed and expected cessation events, and 1-year cumulative incidence of DAPT cessation with corresponding 95%CIs from censoring weighted Kaplan-Meier estimator.
Two-sided P values from weighted log-rank tests comparing the distribution of time to DAPT cessation between the different categories of nonadherence to DAPT.
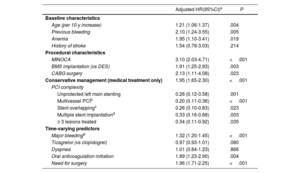
There was a notable overlap between most of the predictors of DAPT cessation and high bleeding risk factors, whereas neither the occurrence of dyspnea nor PY12-i class were predictors of drug cessation (table 4).
Factors associated with premature discontinuation of DAPT
| Adjusted HR(95%CI)a | P | |
|---|---|---|
| Baseline characteristics | ||
| Age (per 10-y increase) | 1.21 (1.06-1.37) | .004 |
| Previous bleeding | 2.10 (1.24-3.55) | .005 |
| Anemia | 1.95 (1.10-3.41) | .019 |
| History of stroke | 1.54 (0.78-3.03) | .214 |
| Procedural characteristics | ||
| MINOCA | 3.10 (2.03-4.71) | <.001 |
| BMS implantation (vs DES) | 1.91 (1.25-2.93) | .003 |
| CABG surgery | 2.13 (1.11-4.08) | .023 |
| Conservative management (medical treatment only) | 1.95 (1.65-2.30) | <.001 |
| PCI complexity | ||
| Unprotected left main stenting | 0.26 (0.12-0.58) | .001 |
| Multivessel PCIb | 0.20 (0.11-0.36) | <.001 |
| Stent overlappingc | 0.26 (0.10-0.83) | .023 |
| Multiple stent implantationd | 0.33 (0.16-0.68) | .003 |
| ≥ 3 lesions treated | 0.34 (0.11-0.92) | .035 |
| Time-varying predictors | ||
| Major bleedinge | 1.32 (1.20-1.45) | <.001 |
| Ticagrelor (vs clopidogrel) | 0.97 (0.93-1.01) | .080 |
| Dyspnea | 1.01 (0.84-1.23) | .866 |
| Oral anticoagulation initiation | 1.89 (1.23-2.90) | .004 |
| Need for surgery | 1.96 (1.71-2.25) | <.001 |
BARC, Bleeding Academic Research Consortium; BMS, bare metal stent; CABG, coronary artery bypass grafting; CI, confidence interval; DAPT, dual antiplatelet therapy; DES, drug-eluting stent; MINOCA, myocardial infarction with nonobstructive coronary arteries; PCI, percutaneous coronary intervention.
Adjusted estimates from multivariate time-updated Cox regression model (details of covariate selection and model diagnostics provided in methods 2 of the supplementary data.
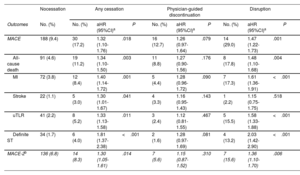
At 1 year, 218 (10%) patients experienced a MACE, of whom 188 (86%) were on DAPT, and 30 (14%) had discontinued treatment before experiencing the event (table 5). Specifically, cardiac events clustered within the first 90 days after discharge (n=106, 48.5%) and declined thereafter. In multivariate models accounting for fixed and time-varying confounders, compared with adherent patients, premature cessation of DAPT was associated with an increased risk of MACE. Differences were largely driven by disruption, whereas physician-guided discontinuation was not associated with a greater risk of MACE (table 5, figure 3). The results remained consistent in direction and magnitude for individual components of the primary endpoint, and with the use of a more restrictive definition of MACE.
Adjusted risk of major adverse cardiac events associated with different patterns of nonadherence to dual antiplatelet therapy within 1 year after acute coronary syndrome
| Nocessation | Any cessation | Physician-guided discontinuation | Disruption | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcomes | No. (%) | No. (%) | aHR (95%CI)a | P | No. (%) | aHR (95%CI)a | P | No. (%) | aHR (95%CI)a | P |
| MACE | 188 (9.4) | 30 (17.2) | 1.32 (1.10-1.76) | .018 | 16 (12.7) | 1.26 (0.97-1.64) | .079 | 14 (29.0) | 1.47 (1.22-1.73) | .001 |
| All-cause death | 91 (4.6) | 19 (11.2) | 1.34 (1.10-1.50) | .003 | 11 (8.8) | 1.27 (0.90-1.56) | .176 | 8 (17.8) | 1.48 (1.10-1.68) | .004 |
| MI | 72 (3.8) | 12 (8.4) | 1.40 (1.14-1.72) | .001 | 5 (4.4) | 1.28 (0.96-1.72) | .090 | 7 (17.3) | 1.61 (1.36-1.91) | <.001 |
| Stroke | 22 (1.1) | 5 (3.0) | 1.30 (1.01-1.67) | .041 | 4 (3.3) | 1.16 (0.95-1.43) | .143 | 1 (2.2) | 1.15 (0.75-1.75) | .518 |
| uTLR | 41 (2.2) | 8 (5.2) | 1.33 (1.13-1.58) | .011 | 3 (2.4) | 1.12 (0.81-1.55) | .467 | 5 (15.5) | 1.58 (1.33-1.88) | <.001 |
| Definite ST | 34 (1.7) | 6 (4.0) | 1.81 (1.37-2.38) | <.001 | 2 (1.6) | 1.28 (0.97-1.69) | .081 | 4 (13.2) | 2.03 (1.42-2.90) | <.001 |
| MACE-2b | 136 (6.8) | 14 (8.3) | 1.30 (1.05-1.61) | .014 | 7 (5.6) | 1.15 (0.87-1.52) | .310 | 7 (15.6) | 1.36 (1.10-1.70) | .006 |
aHR, adjusted hazard ratio; CI, confidence interval; IPCW, inverse probability of censoring weighting; MACE, major adverse cardiac events; MI, myocardial infarction; uTLR, urgent target lesion revascularization; ST, stent thrombosis.
Data are number and 1-year cumulative incidence of events from weighted Kaplan-Meier estimates.
Adjusted hazard ratios with 95%CI from time-updated Cox regression models with DAPT cessation as a time-varying covariate, including doubly robust IPCW estimators and participating hospitals entered as a random-effects variable (Details on multivariate adjustment and model diagnostics provided in the methods 2 of the supplementary data).
Central illustration. DAPT cessation in ACS patients treated with ticagrelor vs clopidogrel. 95%CI, 95% confidence interval; aHR, adjusted hazard ratio; DAPT: dual antiplatelet therapy; IPCW, inverse probability of censoring weighting; MACE, major adverse cardiac events; MI, myocardial infarction; uTLR, urgent target lesion revascularization; ST, stent thrombosis.
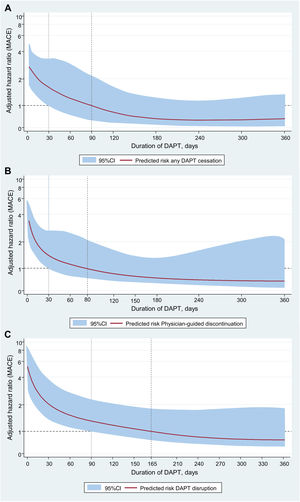
In subgroup analysis, the adjusted risk of MACE after DAPT cessation was significantly higher with ticagrelor than with clopidogrel regardless of cessation mode (Pinteraction<.001) (figure 5 of the supplementary data), particularly during the early postdischarge period (figure 6 of the supplementary data). However, when jointly considering the mode and timing of cessation, this interaction was only evident for DAPT disruption within the first 90 days after discharge (Pinteraction<.001) (figure 7 of the supplementary data). There was a stepwise increase in MACE risk according to the underlying reasons for cessation, with the highest risk noted after noncompliant disruption, particularly within the first 30 days after discharge (figure 8 of the supplementary data, table 5 of the supplementary data). In spline analysis, MACE risk after cessation increased exponentially as DAPT duration shortened, to gradually decrease with prolonged courses of treatment irrespective of cessation mode and the choice of P2Y12-i (figure 4); there were no signals for a time-varying interaction between DAPT cessation and P2Y12-i therapy on MACE risk at any DAPT duration strata (table 5 of the supplementary data, figure 9 of the supplementary data). Likewise, RERI analysis revealed that, compared with clopidogrel, while ticagrelor disruption contributed to a nonsignificant 12% increase in MACE risk, the shorter DAPT regimens resulting from early disruption contributed to a significant increase of 64% in MACE risk associated with cessation (table 6 of the supplementary data). Last, the results of a sensitivity analysis according to PARIS criteria (table 7 of the supplementary data), and in selected datasets (table 8 of the supplementary data) remained consistent with the overall study results.
Duration-response curves depict the aHRs (solid lines in red), and 95%CIs (grey shaded areas) for MACE as a continuous function of DAPT duration. In each panel, the dashed and solid vertical lines plotted over the x-axis denote the duration of continuous DAPT exposure at which the smoothed curves for the aHR and corresponding 95%CI cross the horizontal dashed line of “null effect” (aHR=1). 95%CI, 95% confidence interval; aHR, adjusted hazard ratio; DAPT: dual antiplatelet therapy; IPCW, inverse probability of censoring weighting; MACE, major adverse cardiac events; MI, myocardial infarction; uTLR, urgent target lesion revascularization; ST, stent thrombosis.
The results of this subanalysis of a prospective, multicenter registry of ACS patients intended to receive 12-month DAPT with ticagrelor or clopidogrel can be summarized as follows: a) within 1 year after ACS, nearly 1 in 12 patients prematurely discontinued DAPT; b) overall, physician-guided discontinuation was the most frequent pattern of nonadherence to DAPT; nevertheless, disruption occurred earlier, resulting in shorter courses of treatment; c) DAPT cessation modes significantly varied by P2Y12-i type, with more frequent physician-guided discontinuation among clopidogrel-treated patients, and more disruption in those receiving ticagrelor; d) premature cessation of DAPT was associated with an increased risk of MACE primarily driven by disruption. This risk was highest within 90 days after discharge, with no signal for a modifying effect on this association by the choice of P2Y12-i.
Nonadherence to secondary prevention strategies is a major determinant of treatment effectiveness and successful outcomes for coronary artery disease that remains an important issue in ACS patients.21 Current guidelines have established 12-month DAPT as the standard-of-care after ACS, with potent P2Y12-i conferring greater efficacy over clopidogrel.1 The current benefit of this strategy may translate into a smaller absolute ischemic risk reduction, potentially outweighed by the increased risk of bleeding and higher observed rates of nonadherence with potent P2Y12-i compared with clopidogrel.4,10–12To date, there is limited information on the impact of DAPT cessation with potent P2Y12-i after ACS. In a recent meta-analysis of 4 RCTs the relative risk of premature discontinuation was 25% higher for patients receiving ticagrelor than in those receiving the comparator, mostly driven by bleeding complications followed by dyspnea.4 In observational studies, nonadherence to clopidogrel was almost identical with 20% to 29% of patients treated with clopidogrel stopping DAPT before 12 months,3,22–25 whereas cessation rates with ticagrelor ranged widely between 5% and 30%.8–12,26–29In agreement with a subanalysis of patients with ACS included in the PARIS registry,7 we found that physician-recommend discontinuation was the most common pattern of nonadherence to DAPT. When stratified further, we found that while patient nonadherence was the leading reason for disruption followed by bleeding in the PARIS registry,6 in our study, bleeding complications accounted for more than two-thirds of disruption reasons, with a minority of patients reporting noncompliant disruption. When distinguishing by P2Y12-i, contrary to previous findings,12,29 but similar to that reported by Turgeon et al.,11 we found that DAPT was cessation higher with clopidogrel than with ticagrelor, largely driven by more frequent physician-guided discontinuation. Conversely, in accordance with a recent subanalysis of the Bern PCI registry,10 disruption due to nonadherence and bleeding was more common with ticagrelor than with clopidogrel. In this context, it is noteworthy that noncompliant issues related to twice-daily dosing and medication costs may be important drivers of ticagrelor cessation in daily practice.8–10,12,28 Therefore, we found that, compared with clopidogrel, ticagrelor was discontinued earlier after discharge because of nonadherence, primarily due to a lack of affordability. Interestingly, the median time to DAPT disruption because of nonadherence was about 30 days, which corresponds to the time of the second postdischarge P2Y12-i prescription refill.
The PARIS registry was the first to suggest that cardiac risk after premature DAPT discontinuation varies by underlying reason and timing of cessation.5 Nevertheless, subsequent studies have yielded seemingly conflicting results.3,8–11,22–26 It should be emphasized, however, that much of this previous real-world evidence is based on early studies that may not reflect the contemporary management of ACS in our study. Despite these limitations, the current results closely resemble those from the PARIS registry.5 This demonstrates that, despite substantial advances in the management of ACS since the first report from the PARIS registry, nonadherence to DAPT still remains a primary determinant of treatment success and prognosis in daily practice.
There are several potential reasons that may explain discrepancies between studies, including differences in design, inclusion period, study populations, the definition of nonadherence, and clinical performance across studies. It is noteworthy that, compared with our all-comers cohort, previous reports included selected samples. Combined with the lack of standardization to define and measure medication nonadherence, this seriously hampers the generalizability of the results of previous studies. In contrast, although the current design precludes us from drawing causal associations, the strict follow-up protocol in our study, which included regular telephone interviews and in-person outpatient visits, could have helped identify potential nonadherence behaviors during the early postdischarge period, which would have led to interventions aimed at improving DAPT persistence. This may therefore explain the observed rates of de-escalation from ticagrelor to clopidogrel due to bleeding or dyspnea, which would otherwise have led patients to prematurely discontinue treatment in the current study. The evidence supporting this hypothesis suggests that a delayed outpatient follow-up beyond the first 6 weeks after ACS may result in lower medication adherence and worse short-term outcomes.30 Last, the observed results may reflect the growing concern and awareness regarding potential adverse effects with potent P2Y12-i, with resultant fine-tuning selection of DAPT based on the more favorable trade-off between ischemic and bleeding risk.
Collectively, our findings strongly suggest that timing of DAPT cessation serves a potentially critical role in the subsequent risk of thrombotic events, irrespective of cessation mode and the choice of P2Y12-i after ACS. Of note, in accordance with previous evidence,7,10 we found that this association was nonlinear over time, with a marked excess in thrombotic risk during the first months after discharge. Of note, little research has so far attempted to clarify whether the association between early DAPT cessation and outcomes after ACS are moderated by P2Y12-i class. Interestingly, although the cumulative incidence of MACE after DAPT disruption was higher with ticagrelor than with clopidogrel, after adjusting for exposure duration we found that this association was moderated by the time of discontinuation, irrespective of cessation mode and the choice of P2Y12-i. Most importantly, in accordance with previous studies,6,25 this risk was highest after noncompliant disruption, particularly within the first 30 days after discharge. This finding reinforces the clinical significance of DAPT nonadherence after ACS, and raises serious concerns regarding the use of ultra-short courses of DAPT in high-risk settings.
Against this background, it is currently unknown whether the reversibility of receptor binding and faster offset with ticagrelor compared with clopidogrel might lead to any difference in clinical outcomes after their premature cessation.10–12 The current analysis adds new insights into this controversy by providing no evidence for a differential risk of thrombotic events after unplanned discontinuation of ticagrelor vs clopidogrel at any time point within 12 months after ACS. As a novelty, spline analysis revealed that, compared with clopidogrel, the increased risk of MACE after ticagrelor disruption was largely attributable to the shorter duration of DAPT associated with the particular pattern of nonadherence, rather than a differential prothrombotic effect of ticagrelor cessation itself. In addition, our analysis suggests the possibility of a temporal inflection point around the third month after ACS beyond which the risks associated with DAPT cessation would be negligible. These findings, coupled with those from early pharmacodynamic studies,31 provide reassuring evidence that reversibility of platelet inhibition with ticagrelor vs clopidogrel does not seem to translate into a differential rebound effect in platelet reactivity and resultant greater risk of MACE after their discontinuation. Nevertheless, in view of the observed differential impact of cost-related medication nonadherence according to the P2Y12-i class, efforts should focus on the early identification and prevention of potential nonadherence behaviors due to lack of affordability in ticagrelor-treated patients. Likewise, this study highlights that any step forward to improve patient counselling on the importance of medication adherence, and understanding of the rationale for DAPT may be of paramount importance to minimize nonadherence-related risks after ACS. All these features make the CREA-ARIAM registry (Safety and Effectiveness of Switching Between Antiplatelet Agents, ClinicalTrials.gov Identifier NCT02500290) unique in providing the opportunity to delve more deeply into the clinical significance of DAPT cessation in contemporary daily practice.
LimitationsSome limitations should be also acknowledged. First, relationships between cessation patterns and outcomes cannot be inferred, as we cannot definitely exclude the presence of unmeasured/hidden confounders and potential detection bias. Nonetheless, the results of this study are strengthened by the high level of data granularity collected, which is difficult to achieve in practice in the overwhelming majority of studies, and by the fact that data were prospectively collected independently of treatment and outcomes. Second, nonadherence status was self-reported from patients, which may lead to underreporting because of recall bias. However, to minimize this type of bias, our registry required adjudication of all cessation events by use of any available source documentation. Third, we lacked information on socioeconomic status and medication copayment, which may contribute to medication nonadherence. Nevertheless, this limitation did not seem to significantly affect the study results, given that only a small proportion of patients reported noncompliant disruption because of affordability issues. Last, sensitivity and subgroups analyses were not powered to demonstrate differences across groups and should therefore be considered hypothesis-generating.
CONCLUSIONSIn this contemporary cohort of all-comers with ACS, 1 in 12 patients prematurely discontinued DAPT within 12 months after the index event. Nonadherence to DAPT predicted greater risk of MACE according to the underlying mode and reason for cessation. This association was moderated by DAPT duration, which emerged as the most powerful predictor of cardiac risk after DAPT cessation. The disproportionate excess of thrombotic events after unplanned cessation of ticagrelor compared with clopidogrel was largely attributable to the higher rate of noncompliant disruption and subsequent shorter DAPT duration in the former. These exploratory results warrant further efforts to identify and prevent early DAPT disruption due to nonadherence in ticagrelor-treated patients.
- -
Nonadherence to DAPT remains a major determinant of treatment success and poor outcomes after ACS.
- -
Cardiovascular risk after DAPT discontinuation varies by mode and timing of cessation. However, this evidence is not without limitations given differences in design and the definition of nonadherence across studies.
- -
It is not clear whether cardiac risk after DAPT cessation is moderated by the choice of P2Y12-ihibitor.
- -
In this contemporary multicenter, all-comer registry, 1 in 12 ACS patients prematurely discontinued DAPT.
- -
DAPT cessation was associated with an increased risk of MACE primarily determined by the mode and timing of cessation, with the highest risk noted after noncompliant disruption within 90 days after discharge.
- -
As a novel finding, when we modelled the effect of DAPT cessation on MACE risk as a continuous function of length of exposure, this association was not significantly affected by the choice of P2Y12-ihibitor.
Data collection for this subanalysis was partially supported by an unrestricted research grant (ESR-17–13127) from AstraZeneca Pharmaceuticals Spain, SA, which had no role in study design, collection, analysis, or interpretation of the data, manuscript writing, or in the decision to submit for publication.
AUTHORS’ CONTRIBUTIONSAll authors participated in data acquisition, had access to relevant data, critically revised the manuscript for important intellectual content and participated in the drafting, review, and approval of the final manuscript for submission. M. Almendro-Delia, J.C. García-Rubira and J.A. Arboleda-Sánchez had full access to all data in the study and took responsibility for the integrity of the data and the accuracy of the data analyses. Study concept and design: M. Almendro-Delia, J.C. García-Rubira; analysis and interpretation of data: M. Almendro-Delia, J.C. García-Rubira; blinded-endpoint adjudication: J.C. García-Rubira/M. Almendro-Delia/J.A. Arboleda-Sánchez; drafting of the manuscript: M. Almendro-Delia; study coordinator: M. Almendro-Delia; study supervision: J.C. García-Rubira, and J.A. Arboleda-Sánchez.
CONFLICTS OF INTERESTM. Almendro-Delia has received (modest) honoraria for lectures from Eli Lilly Co, Daiichi Sankyo, and AstraZeneca and reports receiving consulting fees from AstraZeneca and Daiichi Sankyo. The rest of the authors declare no conflicts of interest.