In patients with acute coronary syndrome (ACS) undergoing percutaneous coronary intervention (PCI), nonpersistence with antiplatelet therapy prescribed at discharge may lead to worse outcomes.1 Apart from treatment cessation, nonpersistence may take the form of switching from one agent to another, which is common in everyday clinical practice.2 We present insights from the GReek AntiPlatelet rEgistry (GRAPE) on 1-year nonpersistence with treatment prescribed at discharge.
GRAPE is a prospective, observational, multicenter, cohort study involving consecutive, moderate-to-high risk ACS patients undergoing PCI. Initial P2Y12 receptor antagonist selection along with the subsequent in-hospital and postdischarge antiplatelet agent administration were left to the discretion of the treating clinician. Follow-up was performed at 1, 6, and 12 months by telephone interview or personal contact. Persistence with P2Y12 receptor antagonists was defined as conforming to the recommendation of continuing the same P2Y12 receptor antagonist as that prescribed at discharge. Switching was defined as changing to a different P2Y12 receptor antagonist than that prescribed at discharge, and cessation as not receiving any P2Y12 receptor antagonist.
To assess potential predictive factors for cessation and switching, we used logistic regression modelling and adjusted for type of P2Y12 receptor antagonist, oral anticoagulant, male sex, age (in decades), body mass index (per 5 Kg/m2), diabetes mellitus, hypertension, smoking, reason for admission, prior bleeding, creatinine clearance (calculated by the Cockroft-Gault formula) < 60mL/min, and PCI without stenting or with only bare metal stent use. The model was tested for discriminative power by the C-statistic. Informed consent was obtained from each patient and the protocol was approved by each institution's human research committee. GRAPE has been registered at clinical trials (NCT01774955).
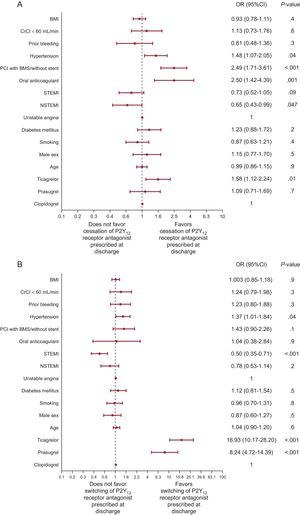
At 1 year, 101 (5%) patients were lost to follow-up, while data on P2Y12 receptor antagonist medication at 1 year were analyzable in 2005 patients. The nonpersistence rate was 24.2% (485 of 2005), with 55.5% (269 of 485) of nonpersistant patients having switched to a different P2Y12 receptor antagonist, while 44.5% (216 of 485) had discontinuated the P2Y12 receptor antagonist. The nonpersistence rate was higher for prasugrel (21.5%) and ticagrelor (37.3%) than for clopidogrel (13.3%), P <. 001 for both, and was higher for ticagrelor than for prasugrel, P < .001. Differences were mainly driven by the higher rate of switching among patients discharged under novel P2Y12 receptor antagonists (2.5%, 13.2%, and 25.0% for clopidogrel, prasugrel, and ticagrelor, respectively), while the cessation rate did not differ among groups (10.9%, 8.3%, and 12.3% for clopidogrel, prasugrel, and ticagrelor, respectively). Out of 269 patients in the switching group, 191 (71.0%) switched from a novel agent (prasugrel or ticagrelor) to clopidogrel, 19 (7.1%) switched from clopidogrel to a novel agent, and 59 (21.9%) switched between novel agents. Patients’ demographic and clinical characteristics are shown in Table. Multivariate predictive models for cessation and switching (Figure) demonstrated fair discriminative power (C-statistic = 0.64; 95% confidencie interval [95%CI], 0.59-0.68; P < .001 and C-statistic = 0.77; 95%CI, 0.74-0.79; P < .001, respectively). Reasons for nonpersistence and 1 year outcomes are provided in the supplementary material.
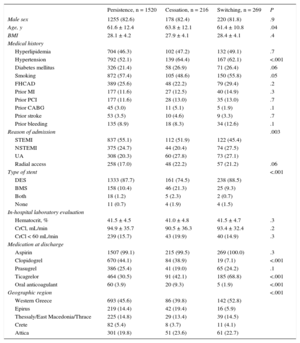
Patients’ Demographic and Clinical Characteristics According to Persistence With Discharge P2Y12 Receptor Antagonist at 1 Year
| Persistence, n = 1520 | Cessation, n = 216 | Switching, n = 269 | P | |
|---|---|---|---|---|
| Male sex | 1255 (82.6) | 178 (82.4) | 220 (81.8) | .9 |
| Age, y | 61.6 ± 12.4 | 63.8 ± 12.1 | 61.4 ± 10.8 | .04 |
| BMI | 28.1 ± 4.2 | 27.9 ± 4.1 | 28.4 ± 4.1 | .4 |
| Medical history | ||||
| Hyperlipidemia | 704 (46.3) | 102 (47.2) | 132 (49.1) | .7 |
| Hypertension | 792 (52.1) | 139 (64.4) | 167 (62.1) | <.001 |
| Diabetes mellitus | 326 (21.4) | 58 (26.9) | 71 (26.4) | .06 |
| Smoking | 872 (57.4) | 105 (48.6) | 150 (55.8) | .05 |
| FHCAD | 389 (25.6) | 48 (22.2) | 79 (29.4) | .2 |
| Prior MI | 177 (11.6) | 27 (12.5) | 40 (14.9) | .3 |
| Prior PCI | 177 (11.6) | 28 (13.0) | 35 (13.0) | .7 |
| Prior CABG | 45 (3.0) | 11 (5.1) | 5 (1.9) | .1 |
| Prior stroke | 53 (3.5) | 10 (4.6) | 9 (3.3) | .7 |
| Prior bleeding | 135 (8.9) | 18 (8.3) | 34 (12.6) | .1 |
| Reason of admission | .003 | |||
| STEMI | 837 (55.1) | 112 (51.9) | 122 (45.4) | |
| NSTEMI | 375 (24.7) | 44 (20.4) | 74 (27.5) | |
| UA | 308 (20.3) | 60 (27.8) | 73 (27.1) | |
| Radial access | 258 (17.0) | 48 (22.2) | 57 (21.2) | .06 |
| Type of stent | <.001 | |||
| DES | 1333 (87.7) | 161 (74.5) | 238 (88.5) | |
| BMS | 158 (10.4) | 46 (21.3) | 25 (9.3) | |
| Both | 18 (1.2) | 5 (2.3) | 2 (0.7) | |
| None | 11 (0.7) | 4 (1.9) | 4 (1.5) | |
| In-hospital laboratory evaluation | ||||
| Hematocrit, % | 41.5 ± 4.5 | 41.0 ± 4.8 | 41.5 ± 4.7 | .3 |
| CrCl, mL/min | 94.9 ± 35.7 | 90.5 ± 36.3 | 93.4 ± 32.4 | .2 |
| CrCl < 60 mL/min | 239 (15.7) | 43 (19.9) | 40 (14.9) | .3 |
| Medication at discharge | ||||
| Aspirin | 1507 (99.1) | 215 (99.5) | 269 (100.0) | .3 |
| Clopidogrel | 670 (44.1) | 84 (38.9) | 19 (7.1) | <.001 |
| Prasugrel | 386 (25.4) | 41 (19.0) | 65 (24.2) | .1 |
| Ticagrelor | 464 (30.5) | 91 (42.1) | 185 (68.8) | <.001 |
| Oral anticoagulant | 60 (3.9) | 20 (9.3) | 5 (1.9) | <.001 |
| Geographic region | <.001 | |||
| Western Greece | 693 (45.6) | 86 (39.8) | 142 (52.8) | |
| Epirus | 219 (14.4) | 42 (19.4) | 16 (5.9) | |
| Thessaly/East Macedonia/Thrace | 225 (14.8) | 29 (13.4) | 39 (14.5) | |
| Crete | 82 (5.4) | 8 (3.7) | 11 (4.1) | |
| Attica | 301 (19.8) | 51 (23.6) | 61 (22.7) | |
BMI, body mass index; BMS, bare metal stent; CABG, coronary artery bypass grafting; CrCl, creatinine clearance; DES, drug-eluting stent; FHCAD, family history of coronary artery disease; MI, myocardial infarction; NSTEMI, non—ST-elevation acute myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; UA, unstable angina.
Values are expressed no. (%) or mean ± standard deviation.
Multivariate analysis of factors affecting cessation (A) and switching (B) assessed at 1 year. 95%CI, 95% confidence interval; BMI, body mass index; BMS, bare metal stent; CrCl, creatinine clearance; NSTEMI, non—ST-elevation acute myocardial infarction; OR, odds ratio; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction.
In GRAPE, at 1 year, differential switching from discharge medication rate was observed among the 3 P2Y12 receptor antagonists, being lowest for clopidogrel. Most importantly, to our knowledge, this report describes for the first time that patients prescribed ticagrelor demonstrate the worst behavior concerning persistence with discharge P2Y12 receptor antagonist, which is driven mainly by the high switching rate. Ticagrelor is the P2Y12 receptor antagonist most recently introduced into clinical practice and is the least well studied outside the setting of clinical trials, although its use is increasing.3 Physician's familiarity, lack of education on the benefit of ticagrelor over clopidogrel or its increase with time, higher cost, twice daily dosing, and adverse effects, among other factors, may be contributory factors.4 More common bleeding events or concern about the higher bleeding potential of novel agents vs clopidogrel may partly explain the better persistence with the latter compared with both novel agents. We identified clinical, eg, hypertension, and treatment characteristics, eg, oral anticoagulant use, PCI with bare metal stent only or without stenting, and ticagrelor prescription at discharge, as factors favoring cessation of discharge P2Y12 receptor antagonists. Moreover, GRAPE provides novel data on factors favoring or discouraging postdischarge switching, namely ticagrelor or prasugrel at discharge and ST-segment elevation myocardial infarction presentation, respectively.
Although there is currently no generally accepted method to define and measure persistence, we used an indirect method—self-reported persistence—which, however, is commonly used and is simple and inexpensive.5 No adjustment was made for the healthy adherer effect. Other factors, eg, level of education, socioeconomic status, stability of family background, which were not included in our predictive model, may also impact on nonpersistence with discharge P2Y12 receptor antagonists and remained unidentified.
Among ACS patients undergoing PCI in settings representative of routine contemporary antiplatelet therapy, 1-year nonpersistence rates differ according to the P2Y12 receptor antagonist prescribed at discharge, being worse for ticagrelor. Early clinical and treatment characteristics may predict P2Y12 receptor antagonist cessation and switching.
CONFLICTS OF INTERESTJ. Goudevenos receives lecture fees by AstraZeneca and D. Alexopoulos receives lecture or advisory fees by AstraZeneca, Boehringer Ingelheim, Bayer, The Medicines Company.