Optical coherence tomography (OCT)-guided percutaneous coronary intervention (PCI) yields clinical outcomes comparable to intravascular ultrasound (IVUS)-guided PCI in patients with stable ischemic heart disease. However, there is a scarcity of data comparing the clinical outcomes of OCT-guided and IVUS-guided PCI in the setting of acute myocardial infarction (AMI). We sought to compare the clinical outcomes of OCT-guided vs IVUS-guided PCI for patients with AMI in the era of second-generation drug-eluting stent (DES).
MethodsWe identified 5260 consecutive patients who underwent PCI with a second-generation DES for AMI under IVUS or OCT guidance from pooled data derived from a series of Korean AMI registries between 2011 and 2020. The primary endpoint was the 1-year rate of target lesion failure, defined as a composite of cardiac death, target vessel myocardial infarction, or ischemia-driven target lesion revascularization.
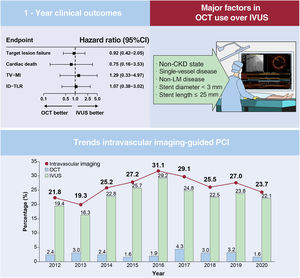
ResultsA total of 535 (10.2%) and 4725 (89.8%) patients were treated under OCT and IVUS guidance, respectively. The 1-year target lesion failure rates were comparable between the OCT and IVUS groups before and after propensity score matching (hazard ratio, 0.92; 95%CI, 0.42-2.05, P=.84). The OCT utilization rate did not exceed 5% of total patients treated with second-generation DES implantation during the study period. The primary factors for the selection of OCT over IVUS were the absence of chronic kidney disease, non-left main vessel disease, single-vessel disease, stent diameter <3mm, and stent length ≤ 25mm.
ConclusionsOCT-guided PCI in patients with AMI treated with a second-generation DES provided comparable clinical outcomes for 1-year target lesion failure compared with IVUS-guided PCI.
Keywords
Intravascular ultrasound (IVUS) and optical coherence tomography (OCT) can depict preintervention lesion characteristics that cannot be visualized using coronary angiography; thus, IVUS and OCT can optimize stent deployment by ensuring a sufficient minimal stent area and stent expansion without edge dissection or stent malapposition.1–5 Therefore, the current European and American guidelines recommend that both IVUS and OCT should be considered for percutaneous coronary intervention (PCI) optimization.6,7 Recent randomized trials have demonstrated the advantages of intravascular imaging, including IVUS- and OCT-guided PCI in patients with complex lesions.8
Several randomized and registry-based studies have demonstrated a significant association between IVUS-guided PCI and a reduction in cardiovascular events in patients with ischemic heart disease.9-11 Recently, 2 dedicated acute myocardial infarction (AMI) registries demonstrated that IVUS guidance improved long-term clinical outcomes.12,13 Moreover, IVUS-guided PCI yielded better clinical outcomes in patients with a high risk of ischemia or chronic kidney disease (CKD) in the setting of AMI, respectively.14,15 Although few studies have directly compared OCT guidance with angiographic guidance,16 randomized trials have demonstrated the non-inferiority of OCT-guided PCI compared with IVUS-guided PCI in patients with ischemic heart disease.17-19 Moreover, a recent meta-analysis also showed comparable outcomes between OCT- and IVUS-guided PCI.20
However, there is a scarcity of data comparing the clinical outcomes of OCT- and IVUS-guided PCI in the setting of AMI. Therefore, this study aimed to investigate the clinical impact of OCT-guided vs IVUS-guided PCI in patients with AMI using a large-scale dedicated AMI registry in the era of second-generation DES.
METHODSStudy design and pooled patient populationThe population of the current study was derived from the Korean Acute Myocardial Infarction Registry-National Institute of Health (KAMIR-NIH) (KCT-0000863)21 and Korean Acute Myocardial Infarction Registry-V (KAMIR-V) (KCT-0008355),22 which are repositories of patients with AMI in the Republic of Korea that do not apply any exclusion criteria. AMI was diagnosed when there was an increased level of cardiac-specific biomarkers, such as troponin I/T or creatinine kinase-MB, with at least 1 value above the 99th percentile upper reference limit and with at least 1 of the following: symptoms of myocardial ischemia, new significant ST-segment-T wave changes, new left bundle branch block, or pathologic Q waves in 2 contiguous leads on a 12-lead electrocardiogram, and imaging evidence of new loss of viable myocardium or new regional wall motion abnormality.23 These registries encompass nationwide, multicenter, web-based, and prospective observational cohorts supported by the Korean Working Group of Acute Myocardial Infarction. The 20 and 43 centers that participated in the KAMIR-NIH and KAMIR-V, respectively, were equipped for primary PCI and on-site cardiac surgery (tables 1 and 2 of the supplementary data). The study protocol was approved by the ethics committees of each participating center. This study complied with the tenets of the Declaration of Helsinki. All patients provided written informed consent to participate in the registry.
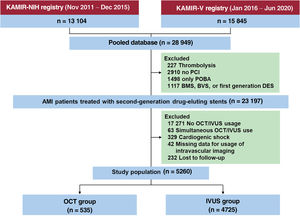
Figure 1 depicts the detailed study flow diagram. We selected 23 197 patients with AMI who underwent PCI with a second-generation DES under IVUS- or OCT guidance from among 28 949 consecutive patients with AMI enrolled between November 2011 and June 2020. The exclusion criteria for the current study were thrombolysis; patients who did not undergo PCI; PCI without stenting; patients treated with a bare-metal stent, first-generation DES, or bioresorbable vascular scaffold; no OCT/IVUS use; simultaneous OCT and IVUS guidance; cardiogenic shock; missing data; and patients lost to follow-up. Patients who were discharged alive but never visited the outpatient department again were designated as “lost to follow-up.” Hence, 5260 patients were selected for this analysis. For the purpose of the present study, participants were divided into an OCT-guided PCI group (n=535) and an IVUS-guided PCI group (n=4725).
Study flowchart. AMI, acute myocardial infarction; BMS, bare-metal stent; BVS, bioresorbable vascular scaffold; DES, drug-eluting stent; IVUS, intravascular ultrasound; OCT, optical coherence tomography; PCI, percutaneous coronary intervention; POBA, plain old balloon angioplasty.
Patients diagnosed with AMI were treated according to contemporary guidelines.24-26 After the diagnosis of AMI, patients routinely received antiplatelet agents including aspirin 300 mg and a P2Y12 inhibitor (clopidogrel 300-600 mg, ticagrelor 180 mg, or prasugrel 60 mg), followed by aspirin (100 mg daily) and P2Y12 inhibitors (clopidogrel 75 mg once, ticagrelor 90 mg twice, or prasugrel 10 mg once daily). The choice of medication was left to each physician's discretion. All procedures were performed in accordance with standard interventional techniques. The decision to use IVUS or OCT during PCI was made at the operator's discretion. Similarly, the treatment strategy, including vascular access, choice of stent, use of thrombus aspiration, and administration of glycoprotein IIb/IIIa inhibitors, was determined by the operator.
Endpoints and definitionsThe primary endpoint was target lesion failure (TLF), defined as a composite of cardiac death, target vessel myocardial infarction (MI), or ischemia-driven target lesion revascularization 1 year after the index procedure. The secondary endpoint included the individual components of TLF, definite/probable stent thrombosis (ST) as defined by the Academic Research Consortium,27 and major adverse cardiovascular events (MACEs), including death from any cause, MI, and revascularization. Target vessel MI was defined as MI with evidence of myocardial necrosis in the vascular territory of a previously treated target vessel. Target lesion revascularization was considered ischemia-driven if any revascularization including PCI or bypass surgery for the target lesion was undertaken in the presence of ≥ 50% angiographic diameter stenosis with ischemic symptoms, positive results on a functional study, or ≥ 70% angiographic diameter stenosis with or without documented ischemia. All-cause mortality was regarded as cardiac death unless a definite non-cardiac cause could be identified. CKD was defined as estimated glomerular filtration rate <60mL/min/1.73 m2 calculated using the Modification of Diet in Renal Disease equation. Acute kidney injury (AKI) was defined as the presence of any of the following (not graded): elevation in the serum creatinine level by ≥ 0.3mg/dL within 48 hours, or increase in serum creatinine level to ≥ 1.5 times that at baseline, whichever was known or assumed to have occurred within the preceding 7 days, or urine volume <0.5mL/kg/h for 6hours.28
Statistical analysisAll data are presented as the mean±standard deviation (SD) for continuous variables and frequency (percentage) for categorical variables. The independent 2 sample t-test was used to compare differences in continuous variables between the 2 groups. The chi-square or Fisher exact test was used to compare differences in categorical variables between the 2 groups depending on the number of events. The mean imputation for missing values was performed to minimize the sample size loss in the analysis. The cumulative incidence rate of the clinical endpoints was estimated using the Kaplan-Meier method. The log-rank test was used to determine whether the cumulative incidence rate of the clinical endpoints differed between the 2 groups. Cox proportional hazard regression analyses were performed to calculate the hazard ratio (HR) with a 95% confidence interval (95%CI) for each clinical endpoint associated with the use of IVUS or OCT. We employed propensity score (PS) matching to account for confounding by indication. Since OCT and IVUS use was not randomized, a PS was used to adjust for selection or predisposition bias. The PS was estimated using multiple logistic regression analysis with all covariates. The OCT and IVUS groups were matched in a ratio of 1:3 without replacement using the nearest-neighbor method based on a PS with a 0.1-caliper width.29 The standardized mean difference was used to assess the balance of covariate distribution between the 2 groups. Covariates with a standardized mean difference <0.1 were considered balanced. We performed univariable and multivariable logistic regression analyses to identify the determinants for the use of OCT. Any variable with a P value of <.10 in the univariate analysis was included in the multivariable models. Data manipulation and statistical analyses were conducted using SAS version 9.3 (SAS Institute) and R software (version 4.1.1; R Foundation for Statistical Computing, Austria). Statistical significance was set at P<.05.
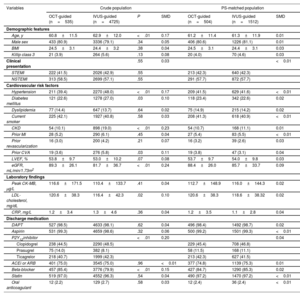
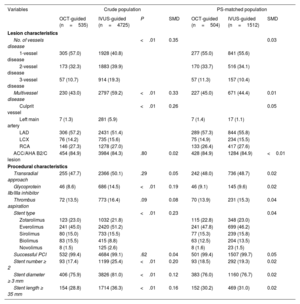
RESULTSBaseline characteristicsThe final analysis included 5260 patients with AMI, of whom 535 (10.2%) underwent OCT-guided PCI and 4725 (89.8%) underwent IVUS-guided PCI with second-generation DES implantation. The mean age of the total study population was 62.7±11.9 years, and 71.7% of the patients were men. Among them, 2248 patients (42.7%) presented with ST-segment elevation myocardial infarction (STEMI). The baseline clinical, lesion, and procedural characteristics of the 2 groups are summarized in table 1 and table 2. The OCT-guided PCI group was younger than the IVUS-guided PCI group. The frequency of hypertension, diabetes mellitus, CKD, and past of history cerebrovascular accident was higher in the IVUS-guided PCI group than that in OCT-guided PCI group. Left ventricular ejection fraction was similar between the 2 groups. Detailed information on medication use in both groups is summarized in table 3 of the supplementary data. The proportion of multivessel disease, culprit lesion located in the left main (LM) artery, and use of glycoprotein IIb/IIIa inhibitors was higher in the IVUS-guided PCI group than that in the OCT-guided PCI group. The IVUS-guided PCI group showed a higher number of implanted stents (1.29±0.52 vs 1.19±0.44; P<.01), a larger implanted stent diameter (3.25±0.48mm vs 3.18±0.47mm; P<.01), and a greater length of the implanted stent (33.7±17.3mm vs 29.3±13.5mm; P<.01) compared with the OCT-guided PCI group. After PS matching, the standardized differences between the groups were<10.0% for all variables, indicating appropriate matching. There were no significant differences in the baseline characteristics between the groups in the PS-matched population (figure 1 of the supplementary data).
Baseline characteristics
| Variables | Crude population | PS-matched population | |||||
|---|---|---|---|---|---|---|---|
| OCT-guided (n=535) | IVUS-guided (n=4725) | P | SMD | OCT-guided (n=504) | IVUS-guided (n=1512) | SMD | |
| Demographic features | |||||||
| Age, y | 60.8±11.5 | 62.9±12.0 | <.01 | 0.17 | 61.2±11.4 | 61.3±11.9 | 0.01 |
| Male sex | 433 (80.9) | 3336 (79.1) | .34 | 0.05 | 406 (80.6) | 1226 (81.1) | 0.01 |
| BMI | 24.5±3.1 | 24.4±3.2 | .38 | 0.04 | 24.5±3.1 | 24.4±3.1 | 0.03 |
| Killip class 3 | 21 (3.9) | 264 (5.6) | .13 | 0.08 | 20 (4.0) | 70 (4.6) | 0.03 |
| Clinical presentation | .55 | 0.03 | <0.01 | ||||
| STEMI | 222 (41.5) | 2026 (42.9) | .55 | 213 (42.3) | 640 (42.3) | ||
| NSTEMI | 313 (58.5) | 2699 (57.1) | .55 | 291 (57.7) | 872 (57.7) | ||
| Cardiovascular risk factors | |||||||
| Hypertension | 211 (39.4) | 2270 (48.0) | <.01 | 0.17 | 209 (41.5) | 629 (41.6) | <0.01 |
| Diabetes mellitus | 121 (22.6) | 1278 (27.0) | .03 | 0.10 | 118 (23.4) | 342 (22.6) | 0.02 |
| Dyslipidemia | 77 (14.4) | 647 (13.7) | .64 | 0.02 | 75 (14.9) | 215 (14.2) | 0.02 |
| Current smoker | 225 (42.1) | 1927 (40.8) | .58 | 0.03 | 208 (41.3) | 618 (40.9) | <0.01 |
| CKD | 54 (10.1) | 898 (19.0) | <.01 | 0.23 | 54 (10.7) | 168 (11.1) | 0.01 |
| Prior MI | 28 (5.2) | 290 (6.1) | .45 | 0.04 | 27 (5.4) | 83 (5.5) | <0.01 |
| Prior revascularization | 16 (3.0) | 200 (4.2) | .21 | 0.07 | 16 (3.2) | 39 (2.6) | 0.03 |
| Prior CVA | 19 (3.6) | 276 (5.8) | .03 | 0.11 | 19 (3.8) | 47 (3.1) | 0.04 |
| LVEF, % | 53.8±9.7 | 53.0±10.2 | .07 | 0.08 | 53.7±9.7 | 54.0±9.8 | 0.03 |
| eGFR, mL/min/1.73m2 | 89.3±26.1 | 81.7±36.7 | <.01 | 0.24 | 88.4±26.0 | 85.7±33.7 | 0.09 |
| Laboratory findings | |||||||
| Peak CK-MB, μg/L | 116.6±171.5 | 110.4±133.7 | .41 | 0.04 | 112.7±148.9 | 116.0±144.3 | 0.02 |
| LDL-cholesterol, mg/dL | 120.6±38.3 | 116.4±42.3 | .02 | 0.10 | 120.6±38.3 | 118.6±38.32 | 0.02 |
| CRP, mg/L | 1.2±3.4 | 1.3±4.6 | .36 | 0.04 | 1.2±3.5 | 1.1±2.8 | 0.04 |
| Discharge medication | |||||||
| DAPT | 527 (98.5) | 4633 (98.1) | .62 | 0.04 | 496 (98.4) | 1492 (98.7) | 0.02 |
| Aspirin | 531 (99.3) | 4659 (98.6) | .32 | 0.06 | 500 (99.2) | 1501 (99.3) | <0.01 |
| P2Y12inhibitor | <.01 | 0.20 | 0.04 | ||||
| Clopidogrel | 238 (44.5) | 2290 (48.5) | 229 (45.4) | 708 (46.8) | |||
| Prasugrel | 75 (14.0) | 382 (8.1) | 58 (11.5) | 168 (11.1) | |||
| Ticagrelor | 218 (40.7) | 1999 (42.3) | 213 (42.3) | 627 (41.5) | |||
| ACEi or ARB | 401 (75.0) | 3545 (75.0) | .96 | <0.01 | 377 (74.8) | 1139 (75.3) | 0.01 |
| Beta-blocker | 457 (85.4) | 3776 (79.9) | <.01 | 0.15 | 427 (84.7) | 1290 (85.3) | 0.02 |
| Statin | 519 (97.0) | 4552 (96.3) | .54 | 0.04 | 490 (97.2) | 1470 (97.2) | <0.01 |
| Oral anticoagulant | 12 (2.2) | 129 (2.7) | .58 | 0.03 | 12 (2.4) | 36 (2.4) | <0.01 |
ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; CKD, chronic kidney disease; CK-MB, creatinine kinase-myocardial band; CRP, C-reactive protein; CVA, cerebrovascular accident; DAPT, dual antiplatelet therapy; eGFR; estimated glomerular filtration rate; IVUS, intravascular ultrasound; LDL, low density lipoprotein; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction; OCT, optical coherence tomography; SMD, standardized mean difference; STEMI, ST-segment-elevation myocardial infarction.
Data are presented as mean±standard deviation or No. (%).
Lesion and procedural characteristics
| Variables | Crude population | PS-matched population | |||||
|---|---|---|---|---|---|---|---|
| OCT-guided (n=535) | IVUS-guided (n=4725) | P | SMD | OCT-guided (n=504) | IVUS-guided (n=1512) | SMD | |
| Lesion characteristics | |||||||
| No. of vessels disease | <.01 | 0.35 | 0.03 | ||||
| 1-vessel disease | 305 (57.0) | 1928 (40.8) | 277 (55.0) | 841 (55.6) | |||
| 2-vessel disease | 173 (32.3) | 1883 (39.9) | 170 (33.7) | 516 (34.1) | |||
| 3-vessel disease | 57 (10.7) | 914 (19.3) | 57 (11.3) | 157 (10.4) | |||
| Multivessel disease | 230 (43.0) | 2797 (59.2) | <.01 | 0.33 | 227 (45.0) | 671 (44.4) | 0.01 |
| Culprit vessel | <.01 | 0.26 | 0.05 | ||||
| Left main artery | 7 (1.3) | 281 (5.9) | 7 (1.4) | 17 (1.1) | |||
| LAD | 306 (57.2) | 2431 (51.4) | 289 (57.3) | 844 (55.8) | |||
| LCX | 76 (14.2) | 735 (15.6) | 75 (14.9) | 234 (15.5) | |||
| RCA | 146 (27.3) | 1278 (27.0) | 133 (26.4) | 417 (27.6) | |||
| ACC/AHA B2/C lesion | 454 (84.9) | 3984 (84.3) | .80 | 0.02 | 428 (84.9) | 1284 (84.9) | <0.01 |
| Procedural characteristics | |||||||
| Transradial approach | 255 (47.7) | 2366 (50.1) | .29 | 0.05 | 242 (48.0) | 736 (48.7) | 0.02 |
| Glycoprotein IIb/IIIa inhibitor | 46 (8.6) | 686 (14.5) | <.01 | 0.19 | 46 (9.1) | 145 (9.6) | 0.02 |
| Thrombus aspiration | 72 (13.5) | 773 (16.4) | .09 | 0.08 | 70 (13.9) | 231 (15.3) | 0.04 |
| Stent type | <.01 | 0.23 | 0.04 | ||||
| Zotarolimus | 123 (23.0) | 1032 (21.8) | 115 (22.8) | 348 (23.0) | |||
| Everolimus | 241 (45.0) | 2420 (51.2) | 241 (47.8) | 699 (46.2) | |||
| Sirolimus | 80 (15.0) | 733 (15.5) | 77 (15.3) | 239 (15.8) | |||
| Biolimus | 83 (15.5) | 415 (8.8) | 63 (12.5) | 204 (13.5) | |||
| Novolimus | 8 (1.5) | 125 (2.6) | 8 (1.6) | 23 (1.5) | |||
| Successful PCI | 532 (99.4) | 4684 (99.1) | .62 | 0.04 | 501 (99.4) | 1507 (99.7) | 0.05 |
| Stent number ≥ 2 | 93 (17.4) | 1199 (25.4) | <.01 | 0.20 | 93 (18.5) | 292 (19.3) | 0.02 |
| Stent diameter ≥ 3 mm | 406 (75.9) | 3826 (81.0) | <.01 | 0.12 | 383 (76.0) | 1160 (76.7) | 0.02 |
| Stent length ≥ 35 mm | 154 (28.8) | 1714 (36.3) | <.01 | 0.16 | 152 (30.2) | 469 (31.0) | 0.02 |
ACC, American College of Cardiology; AHA, American Heart Association; IVUS, intravascular ultrasound; LAD, left anterior descending artery; LCX, left circumflex artery; OCT, optical coherence tomography; PCI, percutaneous coronary intervention; RCA, right coronary artery; SMD, standardized mean difference. Data are presented as mean standard deviation or No. (%).
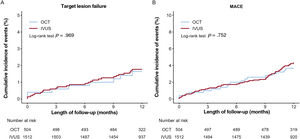
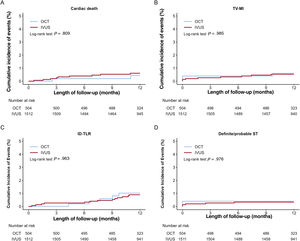
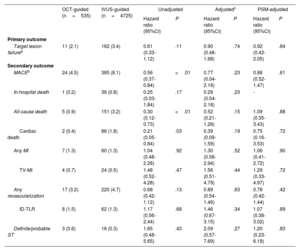
Figure 2 and figure 3, and table 3 present the comparison of the clinical outcomes between the OCT-guided and IVUS-guided PCI groups. The median length of follow-up was 367 [interquartile range, 344 to 387] days. In the study population, 173 TLFs (3.3%; 86 cardiac deaths, 24 target vessel MI, and 70 target lesion revascularizations) occurred after PCI with a second-generation DES for AMI during the 1-year follow-up. TLF at 1 year occurred in 11 patients (2.1%) in the OCT-guided PCI and 162 patients (3.4%) in the IVUS-guided PCI group (HR, 0.61; 95%CI, 0.33-1.12; P=.11). Multiple sensitivity analyses using multivariable Cox regression and PS matching revealed that the risk of MACE, all-cause death, and cardiac death did not differ significantly between the 2 groups, although the unadjusted rate was significantly lower in the OCT-guided PCI group than that in the IVUS-guided PCI group. There was no significant difference in the risk of any MI, target vessel MI, any revascularization, target lesion revascularization, and definite/probable ST between the 2 groups (figure of the supplementary data). For the subgroup analyses, we stratified all patients by age, sex, and important comorbidities. Figure 3 of the supplementary data presents a forest plot showing TLF related to various patient-related or procedural characteristics in the overall population. No significant interaction was observed in the subgroup analyses.
Kaplan-Meier curves for comparison of the rate of 11-year target lesion failure and MACE between OCT-guided and IVUS-guided percutaneous coronary intervention. Target lesion failure (A) and MACE (B). IVUS, intravascular ultrasound; MACE, major adverse cardiac events; OCT, optical coherence tomography.
Kaplan-Meier curves for 1-year cardiac death, TV-MI, ID-TLR, and definite/probable ST.
Cardiac death (A), TV-MI (B), ID-TLR (C), and definite/probable ST (D). ID-TLR, ischemic-driven target lesion revascularization; IVUS, intravascular ultrasound; OCT, optical coherence tomography; ST, stent thrombosis; TV-MI, target vessel myocardial infarction.
One-year outcomes of PCI
| OCT-guided (n=535) | IVUS-guided (n=4725) | Unadjusted | Adjustedc | PSM-adjusted | ||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio (95%CI) | P | Hazard ratio (95%CI) | P | Hazard ratio (95%CI) | P | |||
| Primary outcome | ||||||||
| Target lesion failurea | 11 (2.1) | 162 (3.4) | 0.61 (0.33-1.12) | .11 | 0.90 (0.48-1.68) | .74 | 0.92 (0.42-2.05) | .84 |
| Secondary outcome | ||||||||
| MACEb | 24 (4.5) | 385 (8.1) | 0.56 (0.37-0.84) | <.01 | 0.77 (0.04-2.18) | .23 | 0.88 (0.52-1.47) | .61 |
| In-hospital death | 1 (0.2) | 36 (0.8) | 0.25 (0.03-1.84) | .17 | 0.29 (0.04-2.18) | .23 | - | |
| All-cause death | 5 (0.9) | 151 (3.2) | 0.30 (0.12-0.73) | <.01 | 0.52 (0.21-1.28) | .15 | 1.09 (0.35-3.43) | .88 |
| Cardiac death | 2 (0.4) | 86 (1.8) | 0.21 (0.05-0.84) | .03 | 0.39 (0.09-1.59) | .19 | 0.75 (0.16-3.53) | .72 |
| Any MI | 7 (1.3) | 60 (1.3) | 1.04 (0.48-2.28) | .92 | 1.30 (0.58-2.94) | .52 | 1.06 (0.41-2.72) | .90 |
| TV-MI | 4 (0.7) | 24 (0.5) | 1.48 (0.52-4.28) | .47 | 1.56 (0.51-4.79) | .44 | 1.29 (0.33-4.97) | .72 |
| Any revascularization | 17 (3.2) | 220 (4.7) | 0.68 (0.42-1.12) | .13 | 0.89 (0.54-1.46) | .63 | 0.78 (0.42-1.44) | .42 |
| ID-TLR | 8 (1.5) | 62 (1.3) | 1.17 (0.56-2.44) | .68 | 1.46 (0.67-3.15) | .34 | 1.07 (0.38-3.02) | .89 |
| Definite/probable ST | 3 (0.6) | 16 (0.3) | 1.65 (0.48-5.65) | .43 | 2.09 (0.57-7.69) | .27 | 1.20 (0.23-6.19) | .83 |
ID-TLR, ischemia-driven target lesion revascularization; IVUS, intravascular ultrasound; MACE, major adverse cardiac events; MI, myocardial infarction; OCT, optical coherence tomography; ST, stent thrombosis; TV-MI, target vessel myocardial infarction.
Data are presented as No. (%).
The confounding factors considered in the adjusted HR are age, sex, clinical presentation, hypertension, diabetes mellitus, chronic kidney disease, prior CVA, eGFR, LDL-cholesterol, P2Y12 inhibitor, beta-blocker, multivessel disease, culprit vessel, ACC/AHA B2/C lesion, trans-radial approach, glycoprotein IIb/IIIa inhibitor, thrombus aspiration, stent type, stent number ≥ 2, stent diameter ≥ 3mm, and stent length ≥ 35mm.
The distribution of patients with respect to renal function in the OCT and IVUS groups is presented in table 4 of the supplementary data. The proportion of patients with CKD was higher in the IVUS-guided PCI group than that in the OCT-guided PCI group (898 [19.0%] vs 54 [10.2%], P<.01). In patients with CKD, the incidence rate of postprocedural AKI was not higher in the OCT-guided PCI than in IVUS-guided PCI.
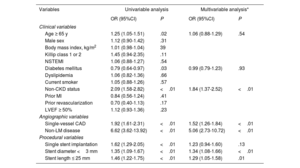
Trends in intravascular imaging-guided PCI and major factors influencing OCT use over IVUSTreatment for AMI was performed under imaging guidance for 25.8% (5989/23 197) of 23 197 patients who underwent PCI with second-generation DES deployment from 2011 to 2020. IVUS-guided PCI was performed in 5350 (23.1%) patients, and 639 (2.8%) patients were treated with OCT-guided PCI (figure 4). The rate of IVUS utilization remained consistently above 20% after 2014; however, the rate of OCT use did not exceed 5% during the study period. We evaluated the major factors influencing OCT use in the current study population using univariable and multivariable logistic regression analyses (table 4). The absence of CKD, non-LM disease, single-vessel disease, stent diameter <3mm, and stent length ≤ 25mm were significant factors associated with the use of OCT over IVUS.
Central illustration. Optical coherence tomography vs intravascular ultrasound-guided percutaneous coronary intervention in patients with acute myocardial infarction. CKD, chronic kidney disease; ID-TLR, ischemia-driven target lesion revascularization; IVUS, intravascular ultrasound; LM, left main artery; OCT, optical coherence tomography; PCI, percutaneous coronary intervention; TV-MI, target vessel myocardial infarction.
Univariate and multivariable analyses of the determinants for OCT use
| Variables | Univariable analysis | Multivariable analysis* | ||
|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | |
| Clinical variables | ||||
| Age ≥ 65 y | 1.25 (1.05-1.51) | .02 | 1.06 (0.88-1.29) | .54 |
| Male sex | 1.12 (0.90-1.42) | .31 | ||
| Body mass index, kg/m2 | 1.01 (0.98-1.04) | 39 | ||
| Killip class 1 or 2 | 1.45 (0.94-2.35) | .11 | ||
| NSTEMI | 1.06 (0.88-1.27) | .54 | ||
| Diabetes mellitus | 0.79 (0.64-0.97) | .03 | 0.99 (0.79-1.23) | .93 |
| Dyslipidemia | 1.06 (0.82-1.36) | .66 | ||
| Current smoker | 1.05 (0.88-1.26) | .57 | ||
| Non-CKD status | 2.09 (1.58-2.82) | <.01 | 1.84 (1.37-2.52) | <.01 |
| Prior MI | 0.84 (0.56-1.24) | .41 | ||
| Prior revascularization | 0.70 (0.40-1.13) | .17 | ||
| LVEF ≥ 50% | 1.12 (0.93-1.36) | .23 | ||
| Angiographic variables | ||||
| Single-vessel CAD | 1.92 (1.61-2.31) | <.01 | 1.52 (1.26-1.84) | <.01 |
| Non-LM disease | 6.62 (3.62-13.92) | <.01 | 5.06 (2.73-10.72) | <.01 |
| Procedural variables | ||||
| Single stent implantation | 1.62 (1.29-2.05) | <.01 | 1.23 (0.94-1.60) | .13 |
| Stent diameter <3 mm | 1.35 (1.09-1.67) | <.01 | 1.34 (1.08-1.66) | <.01 |
| Stent length ≤ 25 mm | 1.46 (1.22-1.75) | <.01 | 1.29 (1.05-1.58) | .01 |
CAD, coronary artery disease; CKD, chronic kidney disease; IVUS, intravascular ultrasound; LM, left main artery; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction.
We compared 1-year clinical outcomes between OCT-guided and IVUS-guided PCI in this large-scale, multicenter cohort study of 5260 patients with AMI, who underwent PCI with second-generation DES implantation. The main findings of the current study are as follows (figure 4): first, there was no significant difference in the 1-year clinical outcomes, including TLF and MACE, between OCT-guided and IVUS-guided PCI. Second, the OCT utilization rate did not exceed 5% of total patients treated with second-generation DES implantation during the study period. Third, the main factors affecting the use of OCT over IVUS were the absence of CKD, non-LM disease, single-vessel disease, stent diameter <3mm, or stent length ≤ 25mm.
Intravascular imaging modalities, such as IVUS, and more recently OCT, have found widespread application in guiding decision-making and optimizing PCI.30 The latest guidelines upgraded the indications for the use of OCT for stent optimization to a Class IIa recommendation, which corresponds to that for IVUS.6,7 In the latest Optical Coherence Tomography Optimized Bifurcation Event Reduction (OCTOBER) trial, OCT-guided PCI demonstrated its superiority in PCI for complex bifurcation lesions by achieving a lower MACE rate at 2 years compared with that of angiography-guided PCI.31 The Optical Frequency Domain Imaging vs Intravascular Ultrasound in Percutaneous Coronary Intervention (OPINION) trials, which included 829 patients, found that the 1-year target vessel failure for OCT was noninferior compared with that for IVUS (5.2% vs 4.9%; P for noninferiority=.04).17 The Optical Coherence Tomography vs Intravascular Ultrasound-Guided Percutaneous Coronary Intervention (OCTVIUS) trial, which involved 2008 patients, demonstrated that the OCT-guided PCI was not inferior to IVUS-guided PCI in TLF at 1 year of follow-up.19 A recent meta-analysis also showed that OCT-guided PCI was associated with reduced all-cause and cardiovascular mortality compared with angiography-guided PCI, and OCT-guided and IVUS-guided PCI yielded comparable outcomes.20 Although no randomized controlled studies have investigated IVUS- vs angiography-guided PCI in the setting of AMI, recent dedicated AMI registries have demonstrated that IVUS guidance improved the long-term clinical outcomes compared with angiography alone.12,13 Moreover, the most recent meta-analysis demonstrated the beneficial effect of IVUS-guided PCI on all-cause mortality (relative risk, 0.70; 95%CI, 0.59-0.82; P<.01), MACE (relative risk, 0.86; 95%CI, 0.74-0.99; P=.04), and target vessel revascularization (relative risk, 0.83; 95%CI, 0.73-0.95; P<.01) in patients with AMI.32 In the current study, we observed that patients who underwent OCT-guided PCI for AMI had a similar risk of the primary outcome, ie, TLF at 1 year, compared with those who underwent IVUS-guided PCI. This could be attributed to the following: in the crude population, the OCT-guided PCI group showed a lower rate of cardiac death than the IVUS-guided PCI group, which was attributed to the younger age and lower prevalence of comorbidities. However, there was no difference between the 2 groups after adjustment for confounding factors. Nevertheless, given the wide confidence intervals for most effect estimates, the findings of the current study were not conclusive, and a larger observational study or randomized trial is still warranted.
Both OCT and IVUS can identify the requisite features for optimal stent implantation including expansion, apposition, and complications, which are not evident on coronary angiography. However, OCT offers limited plaque burden assessment and vessel size detection in the presence of diffuse disease, due to lower tissue penetration, especially in the case of lipid-rich plaque. In contrast, the 10-fold higher resolution of OCT than that of IVUS can facilitate more detailed assessment of plaque morphology, histopathologic features, and stent deployment parameters with a potential clinical impact, including thrombus and culprit plaque identification in patients with AMI, in addition to the detection of residual edge dissection and stent malapposition immediately after stent deployment. The Optical Coherence Tomography-Guided Coronary Stent Implantation Compared to Angiography: a Multicenter Randomised Trial in PCI (ILUMIEN) IV trial showed that OCT guidance resulted in a larger minimal stent area than angiography guidance.33 In addition, the ILUMIEN III trial showed that the post-PCI minimal stent area measured by OCT was noninferior to that of IVUS. The minimum and mean stent expansion with OCT-guided PCI was also comparable to that achieved with IVUS-guided PCI. Untreated major dissections and major malapposition occurred less frequently in the OCT-guided PCI group than in the IVUS-guided PCI group.34 In addition, in the OPINION trial, the in-stent minimum lumen diameter as assessed by quantitative coronary angiography and the rate of binary restenosis at 8 months were comparable between the OCT- and IVUS-guided PCI groups.17 However, previous randomized trials comparing OCT- and IVUS-guided PCI did not exclusively consist of AMI patients. The OPINION trial did not include patients with AMI. In addition, ILUMIEN III trial included only 18% of patients with AMI and excluded STEMI within 24hours of the initial time of presentation. The OCTIVUS trial also included 10% of non-ST-segment elevation myocardial infarction (NSTEMI) patients, excluding STEMI.17,19,34 Therefore, it is important to acknowledge that our study focused on the comparison of the clinical outcomes of OCT and IVUS guidance in a population derived from a dedicated prospective AMI registry in the absence of randomized comparative studies.
The frequency of OCT use for AMI in real-world practice is significantly lower than that of IVUS. In the current study, the rate of IVUS utilization remained consistently above 20% after 2014; however, the rate of OCT use did not exceed 5% in patients treated with second-generation DES during the study period. Similarly, the use rates of IVUS and OCT among patients with AMI who underwent PCI in the United States in 2019 were 8.7% and 0.6%, respectively.35 According to recent surveys, common reasons for reluctance to use intravascular imaging include high cost, prolongation of the procedure, reimbursement policies, uncertainty of its additional clinical benefit, and concerns about adequate training.36,37 The low penetration of OCT in patients with AMI may be attributed to several factors. First, the usefulness of OCT is limited in the presence of high-risk factors for AKI such as CKD because it is necessary to clear the lumen of blood to visualize the vessel wall using additional contrast media.38 Despite this concern, it is not clear whether OCT-guided PCI increases the occurrence of AKI. A recent study showed that OCT-guided PCI did not increase the incidence of AKI compared with IVUS-guided PCI, although increased contrast volume was observed in cases of acute coronary syndrome.39 According to randomized trials comparing OCT- and angiography-guided PCI in patients with NSTEMI, OCT did not increase the incidence of AKI.40 However, in the setting of AMI, especially STEMI, operators perform urgent primary PCI without knowledge of the patient's renal function, which may lead to the preference of IVUS over OCT. Second, OCT-guided PCI is often challenging because of the difficulty of achieving blood clearance due to slow flow during AMI and concerns about the risk of its occurrence. The environment of AMI, which is unfavorable to OCT due to concerns about the use of additional contrast media and slow flow, may be responsible for the low penetration of OCT.
In the present study, the principal factors for the use of OCT over IVUS were absence of renal impairment, non-LM disease, single-vessel disease, stent diameter <3mm, and stent length ≤ 25mm. Current guidelines offer a Class IIa recommendation for IVUS assessment of intermediate LM coronary artery lesions.6,7 Moreover, OCT is underused for LM disease due to the need for blood clearance. In patients with renal impairment or multivessel disease, there are concerns about the possibility of AKI due to the use of additional contrast media during OCT pullback. OCT was used more frequently than IVUS in lesions with a relatively small diameter or short length, where blood clearance was achieved relatively easily. Since the current study was not a randomized trial, we cannot conclusively elucidate that factors that led operators to choose OCT over IVUS. However, this study shows that operators prefer OCT in patients with simple lesions in a single coronary artery and relatively preserved renal function among patients with AMI in real-world practice.
LimitationsThe first limitation of this study its nonrandomized, observational design, which has inherent selection and information biases. Furthermore, there was a large disparity in the number of patients between the two groups. Although a sensitivity analysis with PS matching was conducted to adjust for the measured or unmeasured confounding factors, we cannot exclude the possibility that unmeasured confounders influenced the findings. Second, despite the pooled analyses, differences between centers and operators’ experiences on each imaging modality may affect the findings of the current study. Third, the decision to use IVUS or OCT during PCI was made at the discretion of the operator. Fourth, no detailed procedural data were available on whether postdilation was performed or on the maximum balloon pressure, procedure time, radiation exposure, or total amount of contrast media. Furthermore, there was a lack of comprehensive information on intravascular imaging, including aspects such as the minimal stent area, stent dilatation rate, and acute complications. Additionally, the timing of intravascular imaging relative to the PCI procedure was not addressed. Therefore, the use of intravascular imaging-guided PCI, including IVUS and OCT, cannot guarantee the optimization of PCI, and therefore caution is required in the interpretation of the findings.
CONCLUSIONSOCT-guided PCI provided comparable clinical outcomes in terms of 1-year TLF compared with IVUS-guided PCI. During the study period, OCT penetration was relatively low (< 5%) compared with IVUS in the setting of AMI. Importantly, the factors associated with the use of OCT over IVUS included non-CKD state, non-LM disease, single-vessel disease, stent diameter ˂ 3mm, and stent length ≤ 25mm.
FUNDINGThis research was supported by a fund (2016-ER6304-02) by Research of Korea Centers for Disease Control and Prevention.
ETHICAL CONSIDERATIONSThe study protocol was approved by the ethics committees of each participating center. This study complied with the tenets of the Declaration of Helsinki. All patients provided written informed consent to participate in the registry.
Possible gender biases have been taken into account in the preparation of this article.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCEArtificial intelligence was not used.
AUTHORS’ CONTRIBUTIONSO.H. Lee and Y. Kim designed this study and participated in the final analyses and data interpretation. This report was drafted by O.H. Lee and Y. Kim. All authors approved the final version of the manuscript and ensured that the accuracy or integrity of any part of the work was appropriately investigated and resolved. All authors accessed and O.H. Lee, S.J. Heo, Y. Kim and M.H. Jeong verified the data in the study and had final responsibility for the decision to submit the work for publication.
CONFLICTS OF INTERESTT.W. Johnson has received consultancy and speaker fees from Boston Scientific. The remaining authors have no disclosures to report.
- •
According to real-world data, IVUS-guided PCI improves long-term clinical outcomes in patients with acute myocardial infarction compared with angiography-guided PCI, while several studies showed that OCT-guided PCI yielded outcomes comparable to IVUS-guided PCI in patients with stable ischemic heart disease.
- •
One-year clinical outcomes, including TLF and MACE, did not differ significantly between OCT-guided and IVUS-guided PCI.
- •
The OCT utilization rate did not exceed 5% of the total number of patients treated with second-generation DES implantation during the study period.
- •
The main factors affecting the use of OCT over IVUS were the absence of CKD, non-left main vessel disease, single-vessel disease, stent diameter <3mm, and stent length ≤ 25mm.
The authors would like to thank the clinical investigators of the Korea Acute Myocardial Infarction Registry. The authors wish to thank Medical Illustration and Design, a part of the Medical Research Support Services of Yonsei University College of Medicine, for all artistic support related to this work.