Carbohydrate antigen 125 (CA125) has been shown to be useful for risk stratification in patients admitted with acute heart failure (AHF). We sought to determine a CA125 cutpoint for identifying patients at low risk of 1-month death or the composite of death/HF readmission following admission for AHF.
MethodsThe derivation cohort included 3231 consecutive patients with AHF. CA125 cutoff values with 90% negative predictive value (NPV) and sensitivity up to 85% were identified. The adequacy of these cutpoints and the risk of 1-month death/HF readmission was then tested using the Royston-Parmar method. The best cutpoint was selected and externally validated in a cohort of patients hospitalized from BIOSTAT-CHF (n=1583).
ResultsIn the derivation cohort, the median [IQR] CA125 was 57 [25.3-157] U/mL. The optimal cutoff value was <23 U/mL (21.5% of patients), with NPVs of 99.3% and 94.1% for death and the composite endpoint, respectively. On multivariate survival analyses, CA125 <23 U/mL was independently associated with a lower risk of death (HR, 0.20; 95%CI, 0.08-0.50; P <.001), and the combined endpoint (HR, 0.63; 95%CI, 950.45-0.90; P=.009). The ability of this cutpoint to discriminate patients at a low 1-month risk was confirmed in the validation cohort (NPVs of 98.6% and 96.6% for death and the composite endpoint). The predicted ability of this cutoff remained significant at 6 months of follow-up.
ConclusionsIn patients admitted with AHF, CA125 <23 U/mL identified a subgroup at low risk of short-term adverse events, a population that may not require intense postdischarge monitoring.
Keywords
The first months following hospital admission for acute heart failure (AHF) are characterized by a high risk of death and heart failure (HF) readmissions.1,2 Most of the risk stratification initiatives focus on identifying patients at a higher risk after discharge and less emphasis on recognizing those at a lower risk. Accurate identification of this low-risk population may result in a better allocation of postdischarge resources to high-risk patients.
Plasma levels of carbohydrate antigen 125 (CA125) emerged as a useful prognostic marker in patients with AHF.3–12 Higher CA125 values were shown to be a valuable tool as a proxy of congestion, prognosis, disease monitoring, and tailoring diuretic therapy during hospitalization and the first months after discharge.3–12 Most commercial enzyme-immunoassays recommend 35 U/mL as the cutpoint for defining normal values (). This cutoff was derived from cancer studies carried out in the 1980s.13,14 However, the evidence published on CA125 as a prognostic marker in AHF6,8 showed an exponential risk increase within the range of what is considered normal values. By looking at this stepped sigmoid-shaped risk curve, we hypothesized that the use of lower cutpoints may be useful in identifying patients at a lower risk of early events.
We sought to determine the lowest useful CA125 cutpoints able to identify patients at risk of death and death/HF readmission at 1-month following hospitalization for AHF.
METHODSStudy samplesDerivation cohortWe retrospectively studied a cohort of 3302 consecutive patients admitted for AHF to the Cardiology Department of a third-level teaching center between January 2007 and June 2018. AHF was diagnosed by trained cardiologists according to the definition proposed by the guidelines applicable at the time of patients’ inclusion. On admission, all patients had symptoms or signs attributable to congestion. We included both patients with new onset AHF and decompensated chronic HF. We excluded patients with a missing CA125 value and those who underwent surgical valve replacement, transcatheter valvular intervention during the index admission, or transfer to heart transplant (n=71). The final study sample included 3231 patients.
Left ventricular ejection fraction (LVEF) was assessed by 2-dimensional echocardiography during the index hospitalization. Medical treatment was individualized based on the established guidelines.
Follow-up was limited to 6 months. Patients’ follow-up was censored if death or cardiac transplant (n=2) occurred within this period. The study was approved by an institutional review committee (Hospital Clínico Universitario, Valencia), and patients gave written informed consent. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's human research committee.
BIOSTAT-CHF study (validation cohort)The Biology Study to Tailored Treatment in Chronic Heart Failure (BIOSTAT-CHF) was a multicenter, multinational, prospective, observational study that included 2516 patients with new onset or worsening signs or symptoms of HF from 69 centers in 11 European countries from December 2010 to December 2012.15 Median follow-up was 21 [interquartile range (IQR) 15-27] months. Patients were included after presentation with either new onset or worsening HF, defined as LVEF ≤ 40% and/or brain natriuretic peptide (BNP)> 400 pg/mL or N-terminal pro-B-type natriuretic peptide (NT-proBNP)> 2000 pg/mL. Patients were either seen in the outpatient setting or the hospital. The present study included 1583 hospitalized patients with available CA125 assessments. All patients enrolled in BIOSTAT-CHF gave their written informed consent to participate in the study, and BIOSTAT-CHF was conducted in accordance with the Declaration of Helsinki, national ethics, and legal requirements, as well as relevant European Union legislation. The study was also approved by national and local ethics committees. The characteristics of the BIOSTAT-CHF cohort have been described elsewhere.15
EndpointsAll-cause mortality and the composite of death or HF readmission at 1 and 6 months after hospital admission were selected as endpoints. The information about the cause of death was extracted from the patient's clinical chart and adjudicated by an investigator who was blinded to the values of the exposure.
CA125 measurementIn the derivation cohort, CA125 was measured during hospitalization (48± 24hours after admission) using a commercially available immunoassay kit (Elecsys CA 125 II assay-Roche Diagnostics, Germany). In the validation cohort, CA125 was measured on admission using the ARCHITECT CA 125 II assay (Abbott Laboratories, United States) (lot.81007M800), a chemiluminescent microparticle immunoassay (CMIA), on the ARCHITECTi System (Abbott Laboratories, United States).
The half-life of CA125 is 7 to 12 days, and prior studies have found no significant CA125 changes during the first 72hours following admission.6 The normal value of CA125 established for the assays is 35 U/mL (), and the reported total coefficient of variation is ≤ 10% for all the assays.
Statistical analysisBaseline characteristics among CA125 categories were compared by either ANOVA, Kruskal–Wallis, or chi-square tests, as appropriate.
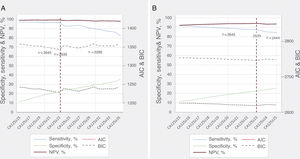
CA125 cutoff for low-risk predictionThe receiver operating characteristic curve (ROC) was estimated for both endpoints at 1 month. To determine the optimal cutoff values of CA125 aimed at identifying low-risk patients, sensitivity, specificity, negative predictive value (NPV), and positive predictive values were estimated along the continuum of the biomarker (diagt command). From a range of cutoffs considered to be predictive of low risk, those with an NPV of 90% or greater, sensitivity of 85% or greater, and those that classified at least 10% of patients as low risk were further analyzed in multivariate survival analyses. In this latter scenario, the best-cutoff was the one with the best performance using the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). Moreover, the prognostic adequacy of the cutoff deemed to be optimal was also tested at 6 months of follow-up. Sensitivity, NPV, AIC, and BIC for the preselected CA125 values are presented in figure 1. The best cutoff was compared with prior reported cutoffs in the literature (traditional cutoff suggested by most of the assays: 35 U/mL, and prognostic cutoffs used in prior studies: 65 U/mL).6–11 For this comparison, patients were classified according to the following CA125 categories: C1, <23 U/mL; C2, 23-34.9 U/mL; C3, 35-64.9 U/mL; and C4, ≥ 65 U/mL.
Performance of different CA125 cutoffs for predicting 1-month adverse events. A: all-cause mortality. B: all-cause mortality/HF readmission. AIC, akaike information criterion; BIC, Bayesian information criterion; CA125, carbohydrate antigen 125; HF, heart failure; NPV, negative predictive value.
Employing a flexible parametric regression model–Royston-Parmar model–we determined the independent prognostic effect of CA125 categories with the 2 clinical outcomes. To hold a linearity assumption with the outcome when modelled as continuous exposure, the best fractional polynomial transformation was chosen based on AIC/BIC criteria. In the derivation cohort, regression estimates from the mortality model were adjusted by age, sex, prior admission for AHF, New York Heart Association (NYHA) before admission, etiology, atrial fibrillation, heart rate, systolic blood pressure, hemoglobin, blood urea nitrogen, NT-proBNP, LVEF, use of beta-blockers during hospital stay, and furosemide equivalent dose on admission. For the composite endpoint, the Charlson comorbidity index and severe tricuspid regurgitation were added to the mortality set of covariates. Under the same multivariate scenario, the prognostic adequacy of the different CA125 cutoffs was also tested.
A similar modelling strategy was applied to the validation cohort, except that these regression estimates were only adjusted with an outcome-specific risk score (BIOSTAT-CHF risk score).16 The BIOSTAT-CHF risk score for mortality included age, blood urea nitrogen, NT-proBNP, serum hemoglobin, and the use of beta-blockers. The BIOSTAT-CHF risk score for the composite endpoint included age, previous HF-hospitalization, peripheral edema, systolic blood pressure, NT-proBNP, hemoglobin, high-density lipoprotein, sodium, and use of beta-blockers. In a sensitivity analysis on the derivation cohort, risk estimates were also adjusted by the appropriate BIOSTAT-CHF risk score for both endpoints.16 Estimates of risk across sex, LVEF (≥ 50% and <50%), estimated glomerular filtration rate (> 60mL/min/1.73 m2 vs ≤ 60mL/min/1.73 m2), and NT-proBNP (above or equal to the median vs below median) were also calculated. All estimates were presented as hazard ratios (HR) with 95% confidence intervals (95%CI). Harrell c-statistics was used as the metric for the model's discrimination performance and presented in figure legends.
We set a 2-sided P-value of <.05 as the threshold for statistical significance. Stata 15.1 (Stata Statistical Software, Release 15 [2017]; StataCorp LP, College Station, United States) was used for the main analysis.
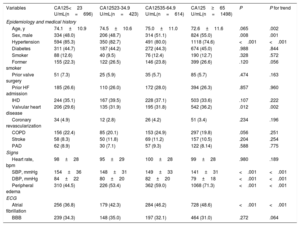
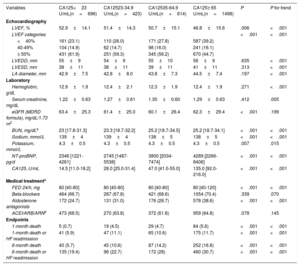
RESULTSThe mean age of the derivation cohort sample was 73.6± 11.3 years; 1553 (48.1%) were female, 861 (26.7%) had a prior hospitalization for AHF, 1142 (35.4%) had a history of ischemic heart disease, and 1697 (52.5%) showed LVEF ≥ 50%. Median [IQR] of CA125, NT-proBNP, and estimated glomerular filtration rate were 57 U/mL [25.3-127], 3418 pg/mL [1831-6918], and 60mL/min/1.73m2 [43.9-76.3], respectively. Baseline characteristics across categories of CA125 are shown in table 1 and table 2. Patients in the lowest category were more frequently females and showed a higher proportion of HF with preserved ejection fraction. Overall, they had a better clinical risk profile (table 1 and table 2). For instance, they showed higher blood pressure, fewer signs of congestion, better renal function, and lower NT-proBNP. Likewise, patients in the lowest category received lower intravenous loop diuretic doses and were less frequently treated with aldosterone receptor antagonists.
Baseline characteristics among CA125 categories. Derivation cohort
| Variables | CA125<23 U/mL(n=696) | CA12523-34.9 U/mL(n=423) | CA12535-64.9 U/mL(n=614) | CA125≥65 U/mL(n=1498) | P | P for trend |
|---|---|---|---|---|---|---|
| Epidemiology and medical history | ||||||
| Age, y | 74.1±10.9 | 74.5±10.6 | 75.0±11.0 | 72.6±11.6 | .065 | .002 |
| Sex, male | 334 (48.0) | 206 (48.7) | 314 (51.1) | 824 (55.0) | .008 | .001 |
| Hypertension | 594 (85.3) | 350 (82.7) | 491 (80.0) | 1118 (74.6) | <.001 | <.001 |
| Diabetes | 311 (44.7) | 187 (44.2) | 272 (44.3) | 674 (45.0) | .988 | .844 |
| Smoker | 88 (12.6) | 40 (9.5) | 76 (12.4) | 190 (12.7) | .328 | .572 |
| Former smoker | 155 (22.3) | 122 (26.5) | 146 (23.8) | 399 (26.6) | .120 | .056 |
| Prior valve surgery | 51 (7.3) | 25 (5.9) | 35 (5.7) | 85 (5.7) | .474 | .163 |
| Prior HF admission | 185 (26.6) | 110 (26.0) | 172 (28.0) | 394 (26.3) | .857 | .960 |
| IHD | 244 (35.1) | 167 (39.5) | 228 (37.1) | 503 (33.6) | .107 | .222 |
| Valvular heart disease | 206 (29.6) | 135 (31.9) | 195 (31.8) | 542 (36.2) | .012 | .002 |
| Coronary revascularization | 34 (4.9) | 12 (2.8) | 26 (4.2) | 51 (3.4) | .234 | .196 |
| COPD | 156 (22.4) | 85 (20.1) | 153 (24.9) | 297 (19.8) | .056 | .251 |
| Stroke | 58 (8.3) | 50 (11.8) | 69 (11.2) | 157 (10.5) | .204 | .254 |
| PAD | 62 (8.9) | 30 (7.1) | 57 (9.3) | 122 (8.14) | .588 | .775 |
| Signs | ||||||
| Heart rate, bpm | 98±28 | 95±29 | 100±28 | 99±28 | .980 | .189 |
| SBP, mmHg | 154±36 | 148±31 | 149±33 | 141±31 | <.001 | <.001 |
| DBP, mmHg | 84±22 | 80±20 | 82±20 | 79±18 | <.001 | <.001 |
| Peripheral edema | 310 (44.5) | 226 (53.4) | 362 (59.0) | 1068 (71.3) | <.001 | <.001 |
| ECG | ||||||
| Atrial fibrillation | 256 (36.8) | 179 (42.3) | 284 (46.2) | 728 (48.6) | <.001 | <.001 |
| BBB | 239 (34.3) | 148 (35.0) | 197 (32.1) | 464 (31.0) | .272 | .064 |
BBB, bundle branch block; CA125, carbohydrate antigen 125; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; ECG, electrocardiogram; HF, heart failure; IHD, ischemic heart disease; PAD, peripheral artery disease; SBP, systolic blood pressure.
Values are expressed as No. (%) or mean±standard deviation.
Complementary examinations, medical treatment and clinical events among CA125 categories. Derivation cohort
| Variables | CA125<23 U/mL(n=696) | CA12523-34.9 U/mL(n=423) | CA12535-64.9 U/mL(n=614) | CA125≥ 65 U/mL(n=1498) | P | P for trend |
|---|---|---|---|---|---|---|
| Echocardiography | ||||||
| LVEF, % | 52.9±14.1 | 51.4±14.3 | 50.7±15.1 | 46.8±15.6 | .006 | <.001 |
| LVEF categories | <.001 | <.001 | ||||
| <40% | 161 (23.1) | 110 (26.0) | 171 (27.8) | 587 (39.2) | ||
| 40-49% | 104 (14.9) | 62 (14.7) | 98 (16.0) | 241 (16.1) | ||
| ≥ 50% | 431 (61.9) | 251 (59.3) | 345 (56.2) | 670 (44.7) | ||
| LVEDD, mm | 55±9 | 54±9 | 55±10 | 56±9 | .635 | <.001 |
| LVESD, mm | 38±11 | 38±11 | 39±11 | 41±11 | .313 | <.001 |
| LA diameter, mm | 42.9±7.5 | 42.8±8.0 | 43.8±7.3 | 44.5±7.4 | .197 | <.001 |
| Laboratory | ||||||
| Hemoglobin, g/dL | 12.9±1.9 | 12.4±2.1 | 12.3±1.9 | 12.4±1.9 | .271 | <.001 |
| Serum creatinine, mg/dL | 1.22±0.63 | 1.27±0.61 | 1.30±0.60 | 1.29±0.63 | .412 | .005 |
| eGFR (MDRD formula), mg/dL/1.73 m2 | 63.4±25.3 | 61.4±25.0 | 60.1±26.4 | 62.3±29.4 | <.001 | .199 |
| BUN, mg/dLa | 23 [17.8-31.3] | 23.3 [18.7-32.2] | 25.2 [18.7-34.5] | 25.2 [18.7-34.1] | <.001 | <.001 |
| Sodium, mmol/L | 139±4 | 139±4 | 138±5 | 138±5 | <.001 | <.001 |
| Potassium, mmol/L | 4.3±0.5 | 4.3±0.5 | 4.3±0.5 | 4.3±0.5 | .007 | .015 |
| NT-proBNP, pg/d | 2346 [1221-4261] | 2745 [1487-5538] | 3800 [2034-7474] | 4289 [2266-8406] | <.001 | <.001 |
| CA125, U/mL | 14.5 [11.0-18.2] | 28.0 [25.0-31.4] | 47.0 [41.0-55.0] | 135.0 [92.0-216.0] | <.001 | <.001 |
| Medical treatmenta | ||||||
| FED 24/h, mg | 80 [40-80] | 80 [40-80] | 80 [40-80] | 80 [40-120] | <.001 | <.001 |
| Beta-blockers | 464 (66.7) | 287 (67.8) | 421 (68.6) | 1054 (70.4) | .339 | .070 |
| Aldosterone antagonists | 172 (24.7) | 131 (31.0) | 176 (28.7) | 578 (38.6) | <.001 | <.001 |
| ACEI/ARB/ARNb | 473 (68.5) | 270 (63.8) | 372 (61.8) | 959 (64.8) | .078 | .145 |
| Endpoints | ||||||
| 1-month death | 5 (0.7) | 19 (4.5) | 29 (4.7) | 84 (5.6) | <.001 | <.001 |
| 1-month death or HF readmission | 41 (5.9) | 47 (11.1) | 65 (10.6) | 175 (11.7) | <.001 | <.001 |
| 6-month death | 40 (5.7) | 45 (10.6) | 87 (14.2) | 252 (16.8) | <.001 | <.001 |
| 6-month death or HF readmission | 135 (19.4) | 96 (22.7) | 172 (28) | 460 (30.7) | <.001 | <.001 |
ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blockers; ARN, angiotensin receptor neprylisin; BUN, blood urea nitrogen; CA125, carbohydrate antigen 125; eGFR, estimated glomerular filtration rate; FED, furosemide equivalent dose; HF, heart failure; LA, left atrial; LVEDD, left ventricle end-diastolic diameter; LVEF, left ventricle ejection fraction; LVESD, left ventricle end-systolic diameter; MDRD, Modification of Diet in Renal Disease; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Values are expressed as No. (%), mean±standard deviation, or median [interquartile range].
The mean age of the validation cohort was 69±12.3 years, 1141 (72.1%) were male, and 425 (26.8%) had a prior hospitalization for AHF. The median [IQR] CA125 was 64 U/mL [21-69], and the distribution and characteristics of patients according to CA125 categories are shown in . Overall, the profile of patients with lower CA125 was similar to that found in the derivation cohort.
CA125 cutoff for selecting low-risk patientsThe area under the ROC curve of CA125 for 1-month death and the composite of death/HF readmission were 0.639 and 0.570, respectively. For 1-month mortality, a range of values from 15 to 34 U/mL were preselected. For the 1-month composite endpoint, a range of values from 15 to 24 U/mL were preselected. Sensitivity, NPV, AIC, and BIC for the preselected CA125 values are presented in figure 1.
A cutoff of 23 U/mL identified 21.5% of the patients with an NPV and sensitivity of 99.3% and 96.4% for 1-month death. The respective diagnostic accuracy measures for the composite endpoint were 94.1% and 87.9%, respectively. After a comprehensive multivariate evaluation, CA125 of 23 U/mL showed the lowest AIC/BIC for both outcomes (figure 1).
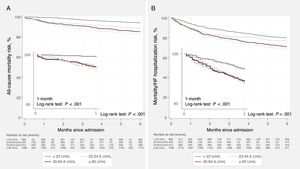
Low CA125 and adverse outcomesDerivation cohortAt 1 month of follow-up, 137 (4.2%) deaths and 328 (10.2%) composite endpoints were registered. Ninety-eight patients (3.0%) died during hospital stay. Crude rates of both endpoints increased when moving from lower to higher CA125 categories (table 1). Kaplan-Meier curves showed a progressive separation of curves throughout the entire follow-up with a tangible separation of curves within the first month for both endpoints and especially for those with CA125 <23 U/mL (figure 2A,B). After a multivariate adjustment, and compared with CA125 ≥ 23 U/mL, those with CA125 <23 U/mL showed an 80% reduction in the risk of death (HR, 0.20; 95%CI, 0.08-0.50; P <.001) and a 37% reduction in the combined endpoint (HR, 0.63; 95%CI, 0.45-0.89; P=.009). A similar risk reduction was also found when compared with patients with CA125 between 23 and 35 U/mL (HR, 0.20; 95%CI, 0.07-0.53; P=.001; and HR, 0.52; 95%CI, 0.34-0.79; P=.002 for death and death/HF readmission, respectively). Survival curves among the prespecified subgroups are presented in figure 1 and . Multivariate analyses revealed a nondifferential association across sex (male vs female), LVEF (≥ 50% vs <50%), NT-proBNP categories [above or equal to median (3480 pg/mL) vs below median], and estimated glomerular filtration rate (> 60mL/min/1.73 m2 vs ≤ 60mL/min/1.73 m2) (figure 3).
CA125 <23 U/mL and risk of adverse events. Multivariate risk estimates. Subgroup analyses. A: all-cause mortality. B: all-cause mortality/HF readmission. 95%CI, 95% confidence interval; AHF, acute heart failure; CA125, carbohydrate antigen 125; eGFR, estimated glomerular filtration rate; HF, heart failure; HR: hazard ratio; LVEF, left ventricle ejection fraction; NT-proBNP, N-terminal pro-B-type natriuretic peptide. For all patients, estimates of risks for mortality were adjusted by age, sex, prior admission for AHF, previous New York Heart Association class before admission, etiology, atrial fibrillation, heart rate, systolic blood pressure, hemoglobin, blood urea nitrogen, NT-proBNP, LVEF, use of beta-blockers during hospitalization, and furosemide equivalent dose on admission. For the composite endpoint, the Charlson comorbidity index and severe tricuspid regurgitation were added to the mortality set of covariates. Harrell's c-statistics for multivariate models for mortality and the composite endpoints were 0.81 and 0.71, respectively.
A sensitivity analysis, adjusting for the BIOSTAT-CHF risk score, also revealed the same pattern of risk for 1-month endpoints. Patients with CA125 <23 U/mL remained associated with a similar lower risk (HR1-monthdeath, 0.17; 95%CI, 0.07-0.41; P <.001; and HR1-monthdeath/HFreadmission, 0.43; 95%CI, 0.31-0.59; P <.001).
At the 6-month follow-up, we registered 424 (13.1%) deaths and 863 (26.7%) combined endpoints. At this time point, the differences indicating a lower risk in patients with CA125 <23 U/mL were of lower magnitude but remained significant for both endpoints (figure 2A,B). Regarding these secondary endpoints, adjusted risk estimates confirmed that CA125 <23 U/mL identified a subgroup of patients with a 51% lower risk of death (HR, 0.49; 95%CI, 0.35-0.69; P <.001) and 22% of death/HF readmission (HR, 0.78; 95%CI, 0.64-0.94; P=.010).
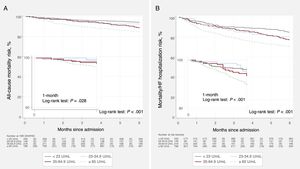
Validation cohortAt 1 month of follow-up, 42 (2.6%) and 120 (7.6%) patients died and experienced the combined endpoint, respectively. Fifty-one (3.2%) patients died during hospitalization. At the 6-month follow-up, 184 (11.6%) patients died, and 371 (23.4%) died or were readmitted for HF. The NPV and sensitivity of CA125 <23 U/mL for 1-month mortality were 98.6% and 85.7%, respectively. The same parameters for 1-month death/HF readmission were 96.6% and 88.3%, respectively. shows the crude rates for 1- and 6-month endpoints across CA125 categories.
Patients with CA125 <23 U/mL had the lowest adverse event rates with differences already present since the first month (figure 4A,B). After multivariate adjustment for the BIOSTAT-CHF risk score, patients with CA125 <23 U/mL continued to show a lower risk when compared with those with CA125> 23 U/mL for 1-month death/HF readmission (HR, 0.54; 95%CI, 0.32-0.91; P=.021). The adjusted risk estimates for 1-month mortality did not reach statistical significance (HR, 0.61; 95%CI, 0.26-1.47; P=.267). At 6 months, CA125 <23 U/mL was associated with a lower risk for both endpoints (HR6-monthdeath, 0.59; 95%CI, 0.39-0.90; P=.014; and HR6-monthdeath/HFreadmission, 0.65; 95%CI, 0.49-0.86; P=.002).
DISCUSSIONThis study confirms the role of CA125 for short- and mid-term risk stratification following a hospitalization for AHF. Moreover, we found that CA125 <23 U/mL (instead of the conventional <35 U/mL) is the optimal cutpoint for identifying patients at lower risk of death at 1 month after hospital admission. The fact that this novel cutpoint (covering 21% of the population) performed equally well for the composite of 1-month death/HF readmission adds robustness to our findings. This cutoff also showed excellent performance for both endpoints measured at the 6-month follow-up. These findings were successfully replicated in hospitalized patients from the BIOSTAT-CHF international cohort of patients with worsening HF.
CA125 in AHF syndromesCA125, also called MUC16, is an extremely complex high molecular glycoprotein, synthesized by coelomic epithelial cells.4,5,17 Although widely used for ovarian cancer monitoring, high plasma levels have been reported in up to two-thirds of patients with AHF.4–6 Although CA125 upregulation mechanisms remain unknown, increased venous pressures and inflammation have been proposed as crucial mechanisms.4,5,17 In AHF, the most important factors positively related to CA125 values are well-known proxies of congestion and right-sided HF.4–8,17,18 Hence, CA125 has gained the attention of clinicians as a surrogate marker of congestion,17 especially given its wide availability, low cost, and limited value of symptoms/signs and natriuretic peptides for accurately measure the severity of congestion.4,5,19,20
Higher CA125 has consistently been shown to be associated with a higher risk of death and HF readmission in different AHF scenarios.3–8 Indeed, a recent substudy of the BIOSTAT-CHF registry confirmed the association of this biomarker with a higher risk of 1-year death and HF readmission independently of traditional prognosticators, including symptoms and signs of congestion.8
Interestingly, this biomarker showed attractive properties for monitoring the first months after decompensation and guiding depletive treatments.9–11 In a recent study in 946 patients discharged with AHF, a longitudinal assessment of CA125 showed that its trajectory in the first weeks after discharge was independently associated with the risk of mortality.9 Furthermore, the CHANCE-HF randomized clinical trial found that a strategy of titrating diuretics and tailoring the intensity of monitoring based on CA125 levels was superior to standard of care in terms of reducing the risk of 1-year death or HF readmission.10 More recently, CA125-guided diuretic therapy was also associated with renal function improvement over usual care in 160 patients with AHF and concomitant renal dysfunction.11
Low CA125, prognosis and potential clinical implicationsTraditional cutpoints for CA125 were derived from studies focused on the diagnostic utility of CA125 for ruling in/out ovarian and other malignancies.13,14,21 For instance, Klug et al.13 found that CA125 <35 U/mL was able to clearly distinguish between healthy individuals and patients with ovarian cancer, while the 65 U/mL cutoff maximized the difference between patients with benign disease and ovarian carcinoma. In other malignancies, CA125> 35 U/mL was also chosen as the most widely accepted cutpoint.21 To date, however, these cancer-driven cutpoints have been used in AHF, with no data on specific cutpoints for AHF. Our findings suggest that a cutpoint at CA125 <23 U/mL indeed identified a low-risk subgroup of patients with a stepwise increase of risk for the immediate upper categories (such as CA125 23-35 U/mL or above). This prognostic effect was seen in both endpoints tested, although it was stronger for mortality, and were consistent in both sexes, in patients with preserved and reduced ejection fraction, renal dysfunction, and independently of NT-proBNP status. Moreover, the prognostic ability of CA125 <23 U/mL was also found at the 6-month follow-up and was positively validated in the BIOSTAT-CHF risk score international cohort. These findings are consistent with prior studies reporting values <20 U/mL in healthy people.14
We envision that our findings may provide a widely available tool for phenotyping the underlying pathophysiology of AHF syndromes. For instance, low CA125 may indicate predominant intravascular congestion or vascular redistribution rather than extravascular or tissue congestion.22 From a clinical point of view, these patients may benefit more from a less intensive diuretic strategy, a more rapid up-titration of recommended standard HF-treatments, and probably more flexible postdischarge monitoring. Finally, these findings reinforce the emerging value of CA125 as a biomarker in HF, as recently stated in a HF expert consensus.23
LimitationsSome limitations need to be acknowledged. First, these results do not apply to patients with stable chronic HF. CA125 was not measured at the same time point in the 2 cohorts. In the derivation cohort, CA125 was measured during the course of hospitalization and on admission in the validation cohort. We believe this issue is irrelevant given the long half-life of this biomarker.4,6,17 Second, we did not evaluate the effect of the intensity of monitoring or the changes in treatments after discharge on the incidence of’outcomes’. Third, since this is a cohort with predominantly Caucasian patients, our findings cannot be extrapolated to other races. Fourth, we did not assess this biomarker at discharge, so the additional value of serial changes of CA125, particularly in patients with long hospitalizations, could not be evaluated with the present approach. Fifth, in the present work, we did not assess the changes in medical treatment that occurred during the follow-up, which may have precluded evaluation of their effect on CA125 status and how they may have modified the result here presented. Consequently, the possible influence of new pharmacological agents such as sacubitril/valsartan and sodium-glucose transporter inhibitors on our findings needs to be evaluated in further works.
CONCLUSIONSIn patients hospitalized for AHF, a cutpoint of CA125 at <23 U/mL identified a subgroup of patients at low risk of short-term mortality and the composite of death/HF-hospitalization. This same cutpoint maintained its predictive ability for both endpoints measured at the 6-month follow-up. Further studies should confirm these findings and evaluate whether this subgroup of patients may receive a more relaxed monitoring schedule during the transitional phase.
FUNDINGThe present work was funded by the European Commission [FP7-242209-BIOSTAT-CHF; EudraCT 2010-020808-29] and CIBER Cardiovascular [16/11/00420 and 16/11/00403].
The funders had no role in the study design, analysis, interpretation of data, nor in the decision to submit the article for publication.
AUTHORS’ CONTRIBUTIONJ. Núñez and A. Bayés-Genís, conceptualization, data analysis, methodology, research, visualization, project management, resources, preparation of the manuscript, and approval of the final version of the manuscript. E. Revuelta-López, G. Miñana, E. Santas, J.M. ter Maaten, R. de la Espriella, Arturo Carratalá, M. Lorenzo, P. Palau, Pau Llàcer, A. Valle, V. Bodi, E. Núñez, J. Lupón, C. Lang, L.L. Ng, M. Metra, J. Sanchis, A.A. Voors, data acquisition, data analysis, research, methodology, visualization, and approval of the final version of the manuscript.
CONFLICTS OF INTERESTThe authors have reported that they have no relevant relationships to the contents of this article to disclose.
- -
The first months after hospitalization for AHF are characterized by a high risk of death and readmissions for AHF.
- -
In patients admitted for AHF, CA125 has been shown to be useful for risk stratification.
- -
Most risk stratification initiatives focus on identifying patients at higher risk after discharge and place less emphasis on recognizing those at lower risk.
- -
Identification of the low-risk population could translate into better resource allocation after discharge.
- -
A new cutoff for CA125 (< 23 U/mL, instead of the conventional <35 U/mL), identified patients with a lower risk of death in the short-term or the combination of death/readmission for heart failure 1 month after discharge after admission for AHF.
- -
The predictive capacity of this cutoff remained significant at the 6-month follow-up.
- -
The identification of this new cutoff allows identification of low-risk patients in the transition phase after admission for AHF.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2021.02.002