ST-segment elevation myocardial infarction (STEMI) is the result of the abrupt occlusion of an epicardial coronary artery. Timely performance of primary percutaneous coronary intervention (PCI) has become the treatment of choice in affected patients. Mechanical reperfusion with implantation of drug-eluting stents (DES) has been associated with improved safety and efficacy compared with balloon angioplasty alone or implantation of bare-metal stents (BMS).1
EARLY GENERATION DRUG-ELUTING STENTSThe Taxus® paclitaxel-eluting stent (PES) (Boston Scientific; Massachusetts, United States) and the Cypher® sirolimus-eluting stent (SES) (Cordis Corporation; New Jersey, United States) were the first early-generation DES widely used in clinical practice. Meanwhile, a wealth of clinical data have shown superior efficacy of early generation DES compared with BMS. A network meta-analysis with a mixed-treatment comparison including 38 trials, in which BMS, PES, and SES were used, reported a lower risk of repeat revascularization (target lesion revascularization) at 4 years of follow-up with both DES compared with BMS, and no differences in mortality. Comparable risks of definite stent thrombosis (ST) (0 days to 4 years) were observed; however, late definite ST (>30 days) was increased with PES compared with BMS or SES. In addition, SES were associated with the lowest risk of myocardial infarction (MI) (hazard ratio [HR]=0.83; 95% confidence interval [95%CI], 0.71-1.00; P=.045 vs PES).2 Along the same line, a meta-analysis of 16 trials comparing SES with PES favored the former in terms of a significant reduction in the risk of reintervention and ST, with a trend toward a higher risk of MI with PES.3
NEW GENERATION DRUG-ELUTING STENTSIn the interim, new generation DES characterized by improved metallic platforms, more biocompatible polymers and new antiproliferative drugs became available. Sirolimus-eluting stents and PES used stainless-steel platforms with relatively thick struts, whereas new DES use cobalt-chromium (ClCr) or platinum-chromium alloys. The latter allowed the design of thin-strut metallic platforms with increased deliverability, visibility, conformability, lower nickel and molybdenum contents, and more rapid reendothelialization. Novel polymers were specifically designed for improved biocompatibility, since polymers of early generation DES were associated with delayed healing and inflammatory reactions. New generation Food and Drug Administration-approved DES include the CoCr Xience V® everolimus-eluting stent (CoCr-EES) (Abbott Vascular; California, United States), and the Endeavor Resolute® zotarolimus-eluting stent (Medtronic; Minnesota, United States), among others.
Currently, more than 40 platforms in different stages of development are being used around the globe. Consecutive innovations include the established superiority of thinner struts, the incorporation of biodegradable polymers as drug carriers, polymer-free drug elution by using micro- or nano-porous surfaces as carriers, exclusive abluminal elution of the drug to prevent toxic endothelial effects, dual-DES with combined antiproliferative and prohealing properties, and even completely bioresorbable scaffolds.4 Randomized clinical evidence has shown that CoCr-EES and Endeavor Resolute® zotarolimus-eluting stent have achieved excellent safety and efficacy outcomes, both in the short- and long-term. CoCr-EES have been associated with the lowest rate of ST, even when compared with BMS.5
COMPARISONS BETWEEN PACLITAXEL-ELUTING STENTS AND EVEROLIMUS-ELUTING STENTSThe SPIRIT family of trials, as well as the all-comer COMPARE trial, has resulted in an extensive body of evidence comparing PES and CoCr-EES. Results from individual trials, subgroup analyses in specific populations, and available registry data are summarized in the Table. In a patient-level pooled analysis of the SPIRIT II, III, IV and COMPARE trials, including 6789 patients, treatment with EES was a powerful, independent predictor of freedom from MI, revascularizations, and major adverse cardiovascular events at 2 years.27 In addition, a meta-analysis of the 3-year follow-up of the SPIRIT trials, showed that EES significantly reduced all-cause mortality compared with PES (3.2% vs 5.1%; P=.003), as well as the risk of definite or probable ST (0.7% vs 1.7%; P=.003).28
Comparison of Early-generation Paclitaxel-eluting Stents and New-generation Everolimus-eluting Stents
| A. RCTs, EES vs PES | SPIRIT II6 | SPIRIT III7 | SPIRIT IV8 | SPIRIT V9 | COMPARE10 | EXECUTIVE11 | PROMISE12 |
| Population characteristics | De novo lesions, noncomplex patients | De novo lesions, noncomplex patients | De novo lesions, noncomplex patients | Diabetic patients | All-comers study | Multivessel CAD | De novo lesions, noncomplex patients |
| Patients, no. | 223 vs 77 | 669 vs 333 | 2458 vs 1229 | 261 vs 115 | 897 vs 903 | 103 vs 97 | 425 vs 425 |
| Angiographic follow-up, months | 6 | 8 | — | 9 | — | 9 | — |
| In-stent late lumen loss, mm | 0.11 vs 0.36a | 0.16 vs 0.30a | — | 0.19 vs 0.39a | — | 0.05 vs 0.24a | — |
| In-stent restenosis, % | 1.3 vs 3.5 | 2.3 vs 5.7 | — | 3.1 vs 6.1 | — | — | — |
| Longest clinical follow-up, months | 60 | 60 | 36 | 24 | 36 | — | 24 |
| MACEb | 8.0 vs 18.1a | 13.7 vs 20.2a | — | 11.2 vs 12.5 | 9.3 vs 14.5a | 11.1 vs 16.5 | 2.5 vs 5.6a |
| Death (cardiac) | 1.5 vs 7.3a | 5.9 vs 10.1a,c | 3.2 vs 5.1a | 0.5 vs 2.9 | 4.9 vs 5.1 | 1.0 vs 1.1 | 1.2 vs 1.5c |
| Myocardial infarction | — | 4.4 vs 6.3 | 3.0 vs 4.6a | 2.8 vs 8.7a,d | 5.2 vs 9.9a | 1.0 vs 3.3 | 0.2 vs 0.8 |
| Target lesion revascularization | 4.7 vs 9.4e | 8.6 vs 12.1e | 6.2 vs 7.8e | 8.4 vs 3.8e | 3.7 vs 7.6a,e | 6.1 vs 7.7e | 1.2 vs 3.5a |
| Definite/probable stent thrombosis | 0.9 vs 2.8 | 1.4 vs 1.6 | 0.6 vs 1.6a | 0.0 vs 1.9 | 1.4 vs 4.9a | — | 0.7 vs 0.3 |
| B. RCTs subgroup analyses, EES vs PES | SPIRIT II and III13 | SPIRIT II and III14 | SPIRIT III15 | SPIRIT III and IV16 | SPIRIT IV17 | SPIRIT IV18 | COMPARE19 |
| Population characteristics | Small vessels (< 2765 mm) | Women | Older patients (≥ 65 years) | Multivessel CAD | Diabetic patients | Jailed side branches | STEMI/NSTEMI |
| Patients, no. | 376 vs 165 | 177 vs 80 | 293 vs 141 | 511 vs 274 | 786 vs 399 | 963 vs 463 | 434 vs 429 |
| Angiographic follow-up, months | 6 and 8 | 6 and 8 | 8 | — | — | — | — |
| In-stent late lumen loss, mm | 0.15 vs 0.30a | 0.09 vs 0.29a | 0.15 vs 0.45a | — | — | — | — |
| In-stent restenosis, % | 2.3 vs 5.7 | 3.1 vs 7.4 | 2.7 vs 12.7a | — | — | — | — |
| Longest clinical follow-up, months | 12 | 24 | 36 | 12 | 12 | 24 | 24 |
| MACEb | 5.2 vs 10.7a | 8.5 vs 16.4a | 7.9 vs 15.4a | 6.2 vs 12.5a | 6.4 vs 7.1 | 6.6 vs 12.2a | 9.2 vs 16.1a |
| Death (cardiac) | 0.8 vs 0.6 | 0.8 vs 0.8 | 1.4 vs 3.0 | 0.8 vs 1.1 | 0.9 vs 0.3 | 1.0 vs 1.4 | 3.0 vs 3.0 |
| Myocardial infarction | 1.9 vs 5.0 | 2.7 vs 7.0a | 3.2 vs 4.6 | 2.2 vs 6.1a,d | 2.6 vs 3.7 | 2.0 vs 5.4a,d | 4.2 vs 8.2a |
| Target lesion revascularization | 3.0 vs 6.3 | 6.2 vs 9.4 | 4.0 vs 7.7 | 4.2 vs 8.0a | 4.2 vs 4.7 | 4.1 vs 7.9a,e | 3.0 vs 6.5a |
| Definite/probable stent thrombosis | 0.3 vs 1.9 | 1.2 vs 0.8 | 1.4 vs 0.0 | 1.2 vs 2.7 | 0.8 vs 1.33 | 1.1 vs 6.3a | 1.4 vs 4.7a |
| C. Registry data, EES vs PES | Taniwaki et al20 | ESTROFA-LM21 | FLM Taxus and LEMAX22 | Valenti et al23 | Simsek et al24 | GHOST25 | ESTROFA-IM26 |
| Population characteristics | Saphenous vein grafts | Left main | Left main | Chronic total occlusions | Diabetic patients | Consecutive patientsf | STEMI |
| Patients, no. | 127 vs 58 | 355 vs 415 | 172 vs 172 | 112 vs 146 | 804 vs 547 | 287 vs 287 | 350 vs 350 |
| Longest clinical follow-up, months | 48 | 36 | 24 | 9 | 36 | 24 | 24 |
| MACEb | 58.7 vs 45.6 | 18.0 vs 16.4 | 7.6 vs 17.4a | 8.9 vs 22.6a | 18.0 vs 20.6 | 13.0 vs 19.0 | 14.9 vs 11.5a |
| Death (cardiac) | 15.3 vs 21.8 | — | 2.3 vs 6.4 | 0.9 vs 2.1 | 12.0 vs 9.6 | 7.0 vs 9.1 | 9.2 vs 9.0c |
| Myocardial infarction | 9.1 vs 1.8 | — | 4.1 vs 9.9a,d | 0.0 vs 2.1 | 3.1 vs 7.4 | 5.6 vs 7.3 | — |
| Target lesion revascularization | 25.8 vs 12.6 | 6.0 vs 4.0 | 4.1 vs 6.4 | 8.0 vs 20.5a,g | 5.6 vs 11.5a | 4.2 vs 8.4a,g | 4.6 vs 2.9 |
| Definite/probable stent thrombosis | 10.1 vs 5.7 | — | 0.6 vs 1.2 | — | 6.8 vs 10.2 | 1.1 vs 3.8a | 4.3 vs 1.4a |
CAD, coronary artery disease; EES, everolimus-eluting stents; MACE, mayor adverse cardiovascular events; NSTEMI, non—ST-segment elevation myocardial infarction; PES, paclitaxel-eluting stents; RCTs, randomized controlled trials; STEMI, ST-segment elevation myocardial infarction.
Analysis of important subgroups (small vessels, women, elderly, multivessel disease, etc.) have corroborated the superiority of EES over PES with the notable exception of diabetic patients, in whom PES resulted in similar clinical outcomes.
VASCULAR HEALING AND AUTOPSY STUDIESImpaired arterial healing is characterized by incomplete reendothelialization, minimal neointimal thickening and persistence of fibrin, and represents an important substrate of late DES thrombosis.29 Intravascular imaging allows the identification of uncovered struts, late acquired malapposition, positive remodeling, and coronary evaginations. Compared with EES, PES have been shown to have a higher frequency of uncovered and malapposed struts (5.2% vs 2.3%, and 5.7% vs 2.1%; P<.001; respectively) in optical coherence tomography investigations at 6 months.30 In another study performed 1 year after implantation of simultaneous PES and EES in 30 patients, PES were significantly associated with a higher proportion of uncovered malapposed struts (62% vs 15%; P<.001), and a larger proximal malapposition area (0.6 [0.3] mm2 vs 0.25 [0.2] mm2; P=.001).31
Along this line, Otsuka et al reported a higher frequency of late and very late ST (26% vs 4%; P<.001), a higher percentage of uncovered struts (18.7% vs 2.6%; P<.001), and higher inflammation scores and fibrin deposition with PES compared with EES in autopsy cases with duration of implantation>30 days and <3 years.32 Interestingly, a recent report comparing 2 devices with the same platform and biodegradable-polymer, but different drug (everolimus or paclitaxel) in a porcine model, has shown lower endothelialization (40 [4%] vs 100%) and higher inflammation scores at 28 days (2.1 [0.3] vs 1.0), as well as a reduced inhibition of neointimal hyperplasia at 90 days with paclitaxel.33
PACLITAXEL-ELUTING STENTS VERSUS EVEROLIMUS-ELUTING STENTS IN ST-SEGMENT ELEVATION MYOCARDIAL INFARCTION PATIENTSIn an article published in Revista Española de Cardiología, de la Torre Hernández et al present the results of a multicenter retrospective registry-based comparison among STEMI patients treated with primary PCI with implantation of either PES or EES. In addition, the authors report a stratified analysis according to the use of thrombectomy.26 Propensity-score matching was used to correct for differences in baseline characteristics in a cohort of 1042 patients, resulting in 2 groups of 350 patients with similar baseline, angiographic, and procedural characteristics.
Retrospective analyses have the drawback that while trying to reduce selection bias (ie, stent selection by the operator), other limitations such as the lack of prospectively defined clinical outcomes, hypotheses and temporal differences become more important; furthermore, unknown confounding factors will always limit the interpretation of propensity-score matching comparisons. For the purpose of this study, 16 centers had to provide 2 different consecutive cohorts of 30 patients, one treated between 2005 and 2007, and the other between 2006 and 2009. The patients had to have all lesions treated with the same stent (either PES or EES) in the setting of primary PCI, while those in cardiogenic shock were excluded.
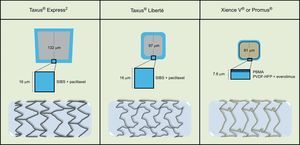
The Taxus® stent has received CE (Conformité Européenne) mark for 3 platforms, namely Taxus® Express2 (January 2003), Taxus® Liberté (September 2005) and Taxus® Element (May 2010). Thus, the first 2 platforms were used in the framework of the present study. In the TAXUS ATLAS trial, 1862 patients were randomized to Taxus® Liberté or Taxus® Express2, and the former achieved noninferiority, with no significant differences in terms of efficacy and safety.34 The Xience V® EES (January 2006) and the Promus® EES (October 2006) are the same stents commercialized under different brand and name (Figure).
Not surprisingly, PES were associated with a higher 2-year incidence of death, MI, and target lesion revascularization (14.9% vs 11.5%; P=.04), as well as definite or probable ST (4.3% vs 1.4%; P=.01) compared with EES. No significant differences were observed in the incidence of all-cause mortality (9.2% vs 9.0%; P=.51). Similar findings were reported in a post hoc analysis of the COMPARE trial, including 864 STEMI and 411 non-STEMI patients, in which a significant reduction of the primary composite endpoint of all-cause death, MI, and target vessel revascularization (9.2% vs 16.1%; P=.002) and definite or probable ST (1.4% vs 4.7%; P=.005) were observed with the use of EES compared with PES at 2 years.19 Notably, the HORIZONS-AMI trial, which randomized 3006 patients to PES or BMS (3:1), showed that the former significantly reduced the 12-month rate of ischemia-driven target lesion revascularization–a benefit that was sustained for 3 years–with no increase in the composite safety outcome of death, reinfarction, stroke, or ST.35
The only direct comparison between CoCr-EES and early-generation DES in acute coronary syndrome patients (96% with STEMI) stems from the XAMI trial,36 in which 625 patients were randomized (2:1) to CoCr-EES or SES. The baseline characteristics reveal a population with a slightly lower risk compared with the current analysis, with less frequent risk factors, previous PCI, and anterior MIs. The primary composite endpoint of cardiac death, MI, and target vessel revascularization was comparable between groups (5.3% vs 4.0%; P=.10), with no differences in ST at 1 year (1.2% vs 2.7%; P=.21). In a comprehensive network meta-analysis including 22 trials using Food an Drug Administration-approved early-generation, new-generation and BMS, Palmerini et al37 reported a lower risk of death or MI with CoCr-EES compared with PES in STEMI patients (HR=0.73; 95%CI, 0.54-0.98), as well as a lower risk of definite or probable ST (HR=0.32; 95%CI, 0.12-0.71). Moreover, CoCr-EES were associated with the most favorable safety and efficacy profile among all devices, including BMS.
INTERACTION BETWEEN STENT PLATFORM AND THROMBUS ASPIRATIONThrombus aspiration in the setting of primary PCI for STEMI remains a matter of debate. The TASTE (Thrombus Aspiration in ST-Elevation Myocardial Infarction in Scandinavia) trial, the largest study published so far comparing the use of aspiration thrombectomy with primary PCI in STEMI patients failed to show a difference in all-cause mortality, reinfarction or target lesion revascularization at 30 days. However, a trend toward a reduction of ST was observed (HR=0.47; 95%CI, 0.20-1.02; P=.06).38 In an updated meta-analysis including 11 321 patient from 20 randomized controlled trials including the TASTE trial, Kumbhani et al have recently reported a significant reduction in late mortality (at 6 to 12 months), as well as lower rates of MI (relative risk=0.64; 95%CI, 0.44-0.92; P=.017) and ST (relative risk=0.54; 95%CI, 0.32-0.91; P=.021).39 De la Torre Hernández et al have found an increased benefit with EES compared with PES in terms of the incidence of ST when thrombus aspiration was not performed. In other words, the use of PES was associated with the highest rates of ST when no thrombectomy was carried out. The ESTROFA (Estudio ESpañol sobre TROmbosis de stents FArmacoactivos) project has yielded interesting contributions to the field of interventional cardiology. In 2008, the first report including 23500 prospectively-included patients revealed a cumulative incidence of ST after early-generation DES of 2% at 3 years, with no differences among PES or SES.40 Two years later, a second report including 4768 patients treated with CoCr-EES or zotarolimus-eluting stent reported a cumulative incidence of definite or probable ST at 2 years of 1.7% for both devices, but a higher rate of ST in patients with bifurcation disease treated with zotarolimus-eluting stent (HR=4.0; 95%CI, 1.1-13.0; P=.03).41 More recently, the SCAAR registry including 61351 unselected patients treated with BMS, early- or new-generation DES, showed that new-generation DES were associated with a significantly lower risk of definite ST compared with BMS (HR=0.38; 95%CI, 0.28–0.52) and early-generation DES (HR=0.57; 95%CI, 0.41–0.79).42
In summary, early generation PES have been largely replaced by new generation DES with a more favorable safety and efficacy profile, which appears to be particularly important in patients with STEMI undergoing primary PCI.
FUNDINGE. Spitzer is the recipient of a research fellowship of the EAPCI (European Association of Percutaneous Cardiovascular Interventions) of the European Society of Cardiology and a research grant of the Spanish Society of Cardiology.
CONFLICTS OF INTERESTS. Windecker has received research contracts to the institution from Biotronik and St. Jude Medical.