Paravalvular leak (PVL) is a complication after valve replacement surgery, with an incidence ranging from 2% to 10% in the aortic position and from 7% to 17% in the mitral position.1 Although most cases have a benign course, 1% to 5% of linked might be linked to serious clinical consequences such as heart failure or hemolytic anemia.1,2 Mortality following re-do surgery has been reported to be high (10%-15%) and rises with the number of previous surgeries.2 Percutaneous treatment of PVL has emerged as a promising alternative to surgery,3 although data comparing the results of surgical vs percutaneous PVL correction are scarce. The purpose of the present study was to describe the outcomes of surgical and percutaneous PVL correction in a contemporary series of patients.
Between January 2006 and December 2015, all patients undergoing isolated PVL through either surgery or the percutaneous approach at our institution were analyzed. The selection of percutaneous or surgical treatment was at the discretion of the treating medical team. However, percutaneous PVL correction became available in 2012 and was performed by experienced operators. Since then, all technically feasible PVLs were initially approached by percutaneous techniques after a Heart Team discussion. To avoid bias, corrective procedures other than isolated PVL, such as combined interventions with coronary revascularization or adjunctive treatment of another cardiac valve, were excluded from the analysis. Patients with active endocarditis were excluded. All patients gave informed consent before the intervention.
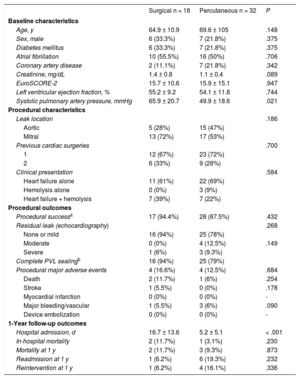
A total of 50 patients (32 percutaneous and 18 surgical) underwent isolated PVL correction and were therefore included in the study (Table). Procedural success was achieved in 94% and 87% of the surgical and percutaneous patients, respectively. Major adverse events and in-hospital mortality were balanced between groups and patients undergoing percutaneous correction had shorter admission periods. At 1-year of follow-up, no significant differences between groups were found in all-cause mortality, hospital readmissions for PVL symptoms, and reintervention (Table).
Baseline, Procedural and Clinical Outcomes Comparing Percutaneous and Surgical PVL Repair
| Surgical n = 18 | Percutaneous n = 32 | P | |
|---|---|---|---|
| Baseline characteristics | |||
| Age, y | 64.9 ± 10.9 | 69.6 ± 105 | .148 |
| Sex, male | 6 (33.3%) | 7 (21.8%) | .375 |
| Diabetes mellitus | 6 (33.3%) | 7 (21.8%) | .375 |
| Atrial fibrillation | 10 (55.5%) | 16 (50%) | .706 |
| Coronary artery disease | 2 (11.1%) | 7 (21.8%) | .342 |
| Creatinine, mg/dL | 1.4 ± 0.8 | 1.1 ± 0.4 | .089 |
| EuroSCORE-2 | 15.7 ± 10.6 | 15.9 ± 15.1 | .947 |
| Left ventricular ejection fraction, % | 55.2 ± 9.2 | 54.1 ± 11.8 | .744 |
| Systolic pulmonary artery pressure, mmHg | 65.9 ± 20.7 | 49.9 ± 18.6 | .021 |
| Procedural characteristics | |||
| Leak location | .186 | ||
| Aortic | 5 (28%) | 15 (47%) | |
| Mitral | 13 (72%) | 17 (53%) | |
| Previous cardiac surgeries | .700 | ||
| 1 | 12 (67%) | 23 (72%) | |
| 2 | 6 (33%) | 9 (28%) | |
| Clinical presentation | .584 | ||
| Heart failure alone | 11 (61%) | 22 (69%) | |
| Hemolysis alone | 0 (0%) | 3 (9%) | |
| Heart failure + hemolysis | 7 (39%) | 7 (22%) | |
| Procedural outcomes | |||
| Procedural successa | 17 (94.4%) | 28 (87.5%) | .432 |
| Residual leak (echocardiography) | .268 | ||
| None or mild | 16 (94%) | 25 (78%) | |
| Moderate | 0 (0%) | 4 (12.5%) | .149 |
| Severe | 1 (6%) | 3 (9.3%) | |
| Complete PVL sealingb | 16 (94%) | 25 (79%) | |
| Procedural major adverse events | 4 (16.6%) | 4 (12.5%) | .684 |
| Death | 2 (11.7%) | 1 (6%) | .254 |
| Stroke | 1 (5.5%) | 0 (0%) | .178 |
| Myocardial infarction | 0 (0%) | 0 (0%) | - |
| Major bleeding/vascular | 1 (5.5%) | 3 (6%) | .090 |
| Device embolization | 0 (0%) | 0 (0%) | - |
| 1-Year follow-up outcomes | |||
| Hospital admission, d | 16.7 ± 13.6 | 5.2 ± 5.1 | < .001 |
| In-hospital mortality | 2 (11.7%) | 1 (3.1%) | .230 |
| Mortality at 1 y | 2 (11.7%) | 3 (9.3%) | .873 |
| Readmission at 1 y | 1 (6.2%) | 6 (19.3%) | .232 |
| Reintervention at 1 y | 1 (6.2%) | 4 (16.1%) | .336 |
PVL, paravalvular leak.
The main findings of the present study were: a) both percutaneous and surgical PVL correction techniques were associated with a high rate of procedural success (> 85%) with a trend toward more complete sealing in patients undergoing surgery; b) in-hospital major adverse events were comparable between groups; c) patients treated with percutaneous techniques had shorter in-hospital admissions; and d) at 1-year of follow-up, clinical outcomes remained balanced between groups.
In our series, surgical patients showed a trend toward more complete PVL sealing but procedural success with percutaneous techniques was still high and similar to that achieved with surgery. These results are in agreement with those of previous publications reporting similar outcomes after percutaneous PVL correction.3 Although our surgical in-hospital mortality might be considered high (11%), it reflects the high surgical risk of the treated population and is in agreement with previous series reporting mortalities between 6% and 22%.1–3 However, it is important to highlight that surgical PVL repair might be the only therapeutic alternative, especially in large or multiple PVLs. Communication within the Heart Team and discussion about the technical complexity of percutaneous repair as well as the proposed surgical strategy is pivotal in these patients. In fact, this communication strategy generally tends to initially offer a percutaneous correction when technically feasible, considering the less invasive nature of the intervention. After a Heart Team discussion, a failed percutaneous PVL correction should therefore not be seen as a “decision mistake” but rather as proof that the move from a less to a more invasive strategy is justified. In addition, surgical techniques might combine additional corrective interventions other than PVL repair that might provide further benefit to the patient. Indeed, in our series, 3 patients with mitral PVLs and percutaneous repair failure underwent corrective mitral surgery and had excellent clinical outcomes. In all 3 cases, the patients and surgeons were informed about the technical complexity of PVL repair beforehand, and surgical repair was conducted during the same hospital admission.
Another relevant finding of the study was the durability of both percutaneous and surgical PVL correction as reflected by the relatively low incidence of 1-year reinterventions and hospital readmissions. Although the relatively small number of patients is a limitation in this regard, some other series seem to confirm this finding.3
In conclusion, our series suggest that percutaneous treatment of isolated PVL seems to be a valid alternative to surgery. Nonetheless, surgical correction should always be considered, as it might be the only option with favorable outcomes in patients with PVLs not suitable for percutaneous repair, after failed percutaneous procedures, or for those patients in need of additional surgical interventions. Larger series will be necessary to confirm these findings.
Conflicts of interestM. Hernández-Enríquez received a training and research grant from the Hemodynamics and Interventional Cardiology Section of the Spanish Society of Cardiology. X. Freixa is a proctor for St Jude Medical.
.