The use of percutaneous left atrial appendage closure (LAAC) for the prevention of stroke in patients with atrial fibrillation is increasing in Spain, where the number of procedures has doubled in just 3 years.1 Preprocedural imaging studies, however, are increasingly detecting atrial thrombosis in candidates for treatment. LAA thrombus has been a contraindication for LAAC in the clinical trials conducted to date. In real-life situations, however, this procedure is necessary in certain patients, despite the increased risk of embolism. Exampes are patients with a history of recurrent embolism despite proper anticoagulant (OAC) therapy (malignant LAA) or bleeding that precludes AOC therapy in the presence of thrombus.
In this study, we determine the prevalence of thrombus in patients referred for LAAC at a high-volume hospital and describe the procedure and associated outcomes. All the patients provided informed consent to be included in the study.
Prior transesophageal echocardiography (TEE) showed LAA thrombus in 8 (10.5%) of the 76 candidates for LAAC. It was decided not to perform the procedure in 3 patients. One had massive thrombosis throughout the appendage and the other 2 were among the first candidates evaluated and it was considered that the operators had not yet acquired sufficient experience. The 5 patients who underwent the procedure all had permanent atrial fibrillation and gave their express consent after being informed in detail about the risks and benefits. The thrombus partly occupied the body of the appendage (the landing zone) in 2 patients and was exclusively located at the tip in the other 3.
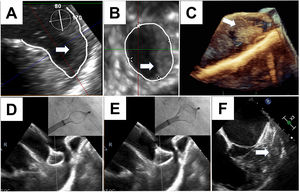
LAAC was performed under general anesthesia, with a number of technical aspects worth noting: a) no materials (eg, guidewires, sheaths, or pigtail catheters) were inserted into the LAA before closure; b) no contrast agents were injected into the LAA; c) the LAAC system was handled very carefully, with close TEE monitoring of the relationship between the sheath, the device, and the LAA; and d) the device was inserted very slowly, in a single maneuver with no repositioning. The main technical considerations for LAAC in the presence of thrombus are illustrated in figure 1. The characteristics of the 5 patients are summarized in table 1.
A-C, thrombos partly occupying the landing zone and tip of the left atrial appendage in patient #5. D and E, progressive deployment of device with echocardiographic guidance (patient #1) and care not to displace the thrombus. F, final position of device forming a cage around the thrombus.
Percutaneous left atrial appendage closure in the presence of thrombus: patient characteristics, anatomic aspects, and antithrombotic treatments
| Patient characteristics | Anatomic aspects and implantation | Antithrombotic treatments | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Age, sex | CHA2DS2-VASc | HAS-BLED | Indication for LAAC | Type of LAA | Location of thrombus | Device | Previous regimens | Before LAAC | After LAAC (1 month) | After LAAC (long-term) |
| 1 | 83 y, F | 7 | 4 | Recurrent stroke on anticoagulants | Windsock | Landing zone and tip | Amulet No. 31 | Acenocumarol, dabigatran, enoxaparin | Apixaban 5 mg/12 h | Apixaban 5 mg/12h+aspirin | Apixaban 5mg/12h |
| 2 | 86 y, F | 7 | 3 | Recurrent stroke on anticoagulants | Chicken wing | Tip | Amulet No. 28 | Acenocumarol | Rivaroxaban 20 mg/24h+aspirin | Rivaroxaban 20mg/24h+aspirin | Rivaroxaban 20mg/24h+aspirin |
| 3 | 83 y, F | 9 | 4 | Stroke on anticoagulants with hemorrhagic transformation | Windsock | Tip | Amulet No. 22 | Acenocumarol, apixaban | Rivaroxaban 20mg/24h+aspirin | Rivaroxaban 20 mg/24h+aspirin | Rivaroxaban 20mg/24h+aspirin |
| 4 | 75, M | 5 | 4 | Recurrent embolism on anticoagulants, Gastrointestinal bleeding | Chicken wing | Tip | Amulet No. 20 | Acenocumarol | Apixaban 5mg/12h | Apixaban 2.5mg/12h | Apixaban 2.5mg/12h |
| 5 | 83 y, F | 5 | 3 | Uncontrollable gastrointestinal bleeding | Windsock | Landing zone and tip | Amulet No. 28 | Acenocumarol, apixaban | Enoxaparin 40mg/24h | Aspirin | None |
CHA2DS2-VASc, score for stroke risk assessment in atrial fibrillation (congestive heart failure, hypertension, age ≥ 75 years [double score], diabetes mellitus, stroke [double score] vascular disease, age 65-74 years, and sex [female]); F, female; HAS-BLED, score for bleeding risk assessment in atrial fibrillation (hypertension, abnormal renal and liver function, stroke, bleeding, labile INR, elderly, drugs or alcohol); LAAC, left atrial appendage closure; M, male.
All the procedures were performed successfully and without complications. Follow-up TEE at 1 month showed no signs of thrombus formation or peridevice leaks. There were no thrombotic events, bleeding, or deaths during a median follow-up of 17.1 months.
Our study is the largest single-center series of LAAC in the presence of thrombosis to be published to date. The good results are consistent with reports from the 2 multicenter studies described so far, in which LAAC was performed successfully in all patients: 28 in the study by Tarantini et al.2 and 27 in that by Boccuzzi et al.3 The thrombi were located exclusively on the tip of the LAA in the cases described by Tarantini et al. Boccuzzi et al. did not specify the location.3 Cerebral protection devices (CPDs) were used in 33 (60.0%) of these 55 patients. CPDs are endovascular filters designed to reduce cerebral embolism in percutaneous aortic values, and based on observational evidence, they appear to be associated with a reduced incidence of stroke.4
While the concept of using CPDs for LAAC in the presence of thrombus is attractive, evidence on their benefits in this setting is lacking. In addition, CPDs may give less experienced operators a false sense of security, but they do not protect against severe embolic events at other locations (eg, coronary, renal, mesenteric, or femoral arteries). In our series, we considered using a CPD in patient #5 because of abundant thrombotic material on the landing zone, but decided against this possibility after detecting severe stenosis of the right carotid artery on an ad hoc arteriogram.
We used Amulet devices (Abbott, USA) in all cases, as these are the devices with which we have the most experience. Other systems, however, have been described.
Our findings indicate that LAAC in the presence of thrombus is feasible when performed by an experienced operator using a suitable technique and proper echocardiographic guidance. Systematic CPD use is not necessary. LAAC should therefore be considered in selected patients with LAA thrombus despite AOC therapy or in patients with a history of major bleeding.
FundingNone.
Authors’ ContributionsA Fontenla conceived the idea for the study; A Fontenla, C Corros-Vicente, I Gómez-Blázquez, and R Arboleda-Salazar collected the data; A Fontenla and R Arboleda-Salazar wrote the manuscript; A Fontenla, C Corros-Vicente, and I Gómez-Blázquez prepared the figure; and F Arribas and R Salguero-Bodes critically reviewed the manuscript.
Conflicts of InterestNone declared.