Mitral regurgitation is the most prevalent valvular heart disease worldwide. Despite the widespread availability of curative surgical intervention, a considerable proportion of patients with severe mitral regurgitation are not referred for treatment, largely due to the presence of left ventricular dysfunction, advanced age, and comorbid illnesses. Transcatheter mitral valve replacement is a promising therapeutic alternative to traditional surgical valve replacement. The complex anatomical and pathophysiological nature of the mitral valvular complex, however, presents significant challenges to the successful design and implementation of novel transcatheter mitral replacement devices. Patient-specific 3-dimensional computer-based models enable accurate assessment of the mitral valve anatomy and preprocedural simulations for transcatheter therapies. Such information may help refine the design features of novel transcatheter mitral devices and enhance procedural planning. Herein, we describe a novel medical image-based processing tool that facilitates accurate, noninvasive assessment of the mitral valvular complex, by creating precise three-dimensional heart models. The 3-dimensional computer reconstructions are then converted to a physical model using 3-dimensional printing technology, thereby enabling patient-specific assessment of the interaction between device and patient. It may provide new opportunities for a better understanding of the mitral anatomy-pathophysiology-device interaction, which is of critical importance for the advancement of transcatheter mitral valve replacement.
Keywords
Mitral regurgitation (MR) is increasingly prevalent, affecting up to 9% of individuals aged 75 years or older.1,2 Although surgery is the gold standard treatment for severe MR, approximately half of all patients with indications for mitral valve (MV) surgery are denied or not referred for potentially curative intervention, mainly due to advanced age, substantial comorbidities, or left ventricular dysfunction, which make surgical risk unacceptably high.3,4 Moreover, there is currently little evidence to support surgical intervention in patients with functional MR.5 Therefore, there is a considerable unmet clinical need for a less invasive therapeutic option for a large proportion of patients with MR.
Transcatheter aortic valve replacement has demonstrated the safety and efficacy of transcatheter heart valve interventions.6–8 In contrast, the development of transcatheter MV replacement (TMVR) devices is in its infancy. Acute and chronic animal studies of TMVR have shown encouraging results,9,10 and more recently, several first-in-man procedures have been performed with a variety of prostheses.11,12 TMVR lags behind its aortic equivalent due to the complex and highly variable anatomy of the MV, the interplay of the valve with the left ventricle, and the highly variable effect of different pathologies (primary and secondary MR) on the mitral apparatus.
A better understanding of the mitral anatomy-pathophysiology-device interaction is of critical importance for the advancement of the field of TMVR. The impact of multislice computed tomography on transcatheter aortic valve replacement outcomes validates this concept,13 though the more complex anatomy of the MV potentially necessitates dedicated cardiac imaging technology. Novel imaging software packages can create accurate patient-specific 3-dimensional (3D) heart models for 3D printing. The advantages of this technology include: a) enhanced design of valve prototypes; b) improved preprocedural patient selection; c) intraprocedural guidance; and d) post-procedure clinical surveillance and understanding of patient-prosthesis interaction.
The aim of this review is to illustrate the potential of 3D computer-based models of the MV using Mimics© Innovation Suite software (Materialise NV, Leuven, Belgium). We show how this software can be used to better understand the anatomy of the MV (anatomy, position and relationships), and to develop virtual simulations and 3D printing for optimizing TMVR.
MIMICS© INNOVATION SUITE SOFTWARE PACKAGEThe key to converting anatomical data from 2D imaging modalities (echocardiography, fluoroscopy, and planar images from multislice computed tomography or magnetic resonance imaging) to 3D models is a process called segmentation. The reconstructed 3D heart computer models can then be 3D-printed in its entirety or limited according to the structures of interest (eg, only left-sided heart chambers). Various materials and 3D printing technologies are available (stereolithography, laser sintering, polyjet, powder binding, etc) and afford the opportunity to produce a 3D model for a specific purpose: understanding anatomy, prosthesis design, prosthesis testing, and procedural planning.
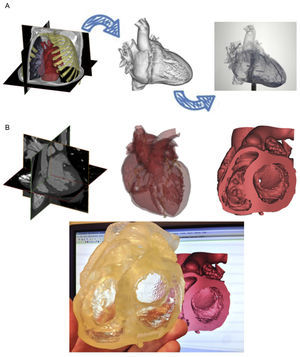
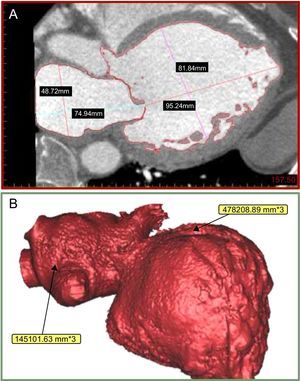
The Mimics© Innovation Suite software (Materialise NV) is a specialized program that enables the processing of multislice computed tomography data. This software package incorporates a multitude of operations, from simple measurements to complex features such as 3D modeling, prosthesis design, and complex 3D measurement functionality. It permits the creation of precise 3D heart models to facilitate patient-specific anatomical assessment and understanding of the interaction between an individual patient's anatomy and a specific prosthesis design (Figures 1A and B).
A: Mimics© Innovation Suite software package permits the creation of accurate 3D models from 2D imaging data. B: Mimics© software (Materialise NV) permits the segmentation of data from multislice computed tomography of the specific area of heart (MV complex) of an individual patient. Thus, we can create accurate 3D models of the MV complex and ultimately print them in a variety of materials and colors.
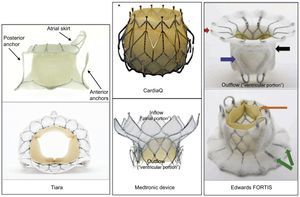
Transcatheter MV device design is recognized to be an extremely challenging process. This complexity arises from: a) the requirement to anchor the prosthesis at the level of the mitral annulus in the absence of a circumferential rigid annulus or leaflet calcification; b) the bulky and highly individualized nature of the submitral valvular apparatus (papillary muscles and chordae tendinae) which represents a physical obstacle for device delivery, deployment, alignment, and expansion; c) the combination of higher closing pressures (ie, systolic) and large orifice area suggests higher dislodgement forces12; d) the variability in both MV pathology (functional and degenerative regurgitation, stenosis) and annular dimensions suggests that there is a need for multiple prosthesis designs and sizes; e) the anatomical association between the mitral annulus and the left ventricular outflow tract (LVOT) could potentially yield LVOT obstruction; f) the location of the left circumflex coronary artery could limit radial expansion; g) the large annulus diameter and requirement for a device retrieval mechanism necessitate large bore delivery catheters (> 25 Fr); and h) the dimensions of the left ventricle or atrium potentially limit room for maneuvering the large catheters required for prosthesis deployment.
Mitral Valve AnatomyThe mitral annulus refers to the transition between the left atrium and left ventricle, which gives rise to the basal attachment of the MV leaflets. The MV annulus is a D-shaped structure with the intercommissural (septolateral) diameter being larger than the anteroposterior diameter.14 It is fibrous, pliable and rarely calcified and is characterized by dynamic changes in mitral annular geometry (shape/size) during the cardiac cycle (reduction in annular area (30%) and annular circumference (15%) during ventricular systole.10 The aortic (anterior) mitral leaflet is in fibrous continuity with the aortic valve and the right and left fibrous trigones. This region of the annulus is thus fibrous and less prone to dilatation. The remaining two-thirds of the annulus are mainly muscular.
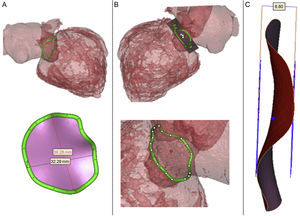
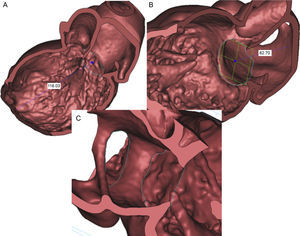
As shown in Figures 2A and 2B, the Mimics© Innovation Suite automatically projects the 3D annulus onto its best-fit plane and shows the geometric center of the MV annulus before the annular area and perimeter are measured automatically. The mean diameter is calculated as the arithmetic mean of the aorto-mural and intercommissural.10 These measurements are important because, similarly to transcatheter aortic valve replacement, accurate and precise sizing of the mitral annulus may be a key issue in selecting the appropriate device size in TMVR. In contrast to transcatheter aortic valve replacement in which radial force is used for anchoring, TCMVR rely on axial fixation (Figure 3). A large inflow atrial portion and short left ventricular outflow portion of the fixation system are designed to mitigate the risk of LVOT obstruction. Excessive oversizing may result in annular rupture or LVOT obstruction with suboptimal cardiac output. Undersizing may result in paravalvular leak and/or embolization.
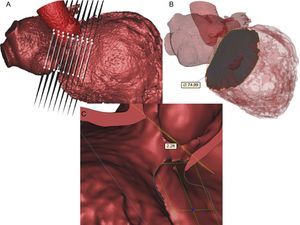
These are the muscular components of the mitral apparatus. As a functional unit, the papillary muscles include a portion of the adjacent left ventricular wall. They are generally described in anterolateral and posteromedial positions and are positioned along the mid to apical segments of the left ventricle. However, this distribution can vary significantly, particularly in patients with myxomatous-type leaflets.15 Measurements performed in Mimics© are taken from a point on each papillary muscle head to the geometric center of MV annulus (Figure 4A) and MV plane (perpendicular to plane) (Figure 4B). The 3D linear distance between the geometrical center and each papillary muscle head and the perpendicular distance between the annulus best-fit plane and each papillary muscle head can be measured (Figure 4C). Mimics© allows rendering of 3D and cross-sectional anatomy permitting optimal visualization of the number and location of papillary muscles (Figure 4D).
Papillary muscle. A: Measurement taken from point on papillary muscle heads to the geometric center of mitral valve annulus. B: Measurement acquired from papillary muscle heads to mitral plane (perpendicular to plane). C: Linear distance between tips of papillary muscles. D: Flexibility to render and cut anatomy to obtain optimal visualization of papillary muscles (number of papillary muscles).
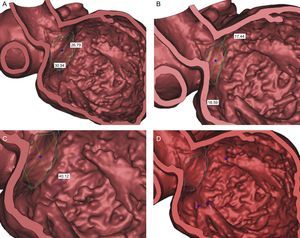
Prosthesis size, shape, and anchorage interact not only with the mitral annulus, but may also interact with anatomical structures on the atrial and/or ventricular sides of the annulus. The left atrium and ventricle measurements aim to describe the shape of the cavity and, most importantly, define in detail the region just beneath and above the mitral annulus, including the LVOT. Using Mimics©, we can measure accurate left ventricular and atrial diameters and volumes (Figures 5A and B). The left ventricular long-axis diameter is defined as the distance between the mitral annulus geometrical center and the left ventricular apex and left atrial roof, respectively (Figures 6A and B). In addition, the software permits analysis of the relationship between left atrial appendage ostia and the MV annulus (Figure 6C). The left atrium and ventricle can also be analyzed at predetermined increments (eg, 5mm) from the mitral annulus to better understand its area and perimeter at these levels and the possible interactions with prostheses (Figure 7A and B). Accurate study of the region just beneath the mitral annulus is of particular interest since it may constitute a landing-zone for TMVR prostheses. To assess the important relationship between the mitral annulus and the LVOT, several measurements are of particular interest in the context of TMVR. The aorto-mitral angle, which lies between the axis of the LVOT and the centerline of the mitral annulus, is noted. The distance from the medial aspect of the mitral annulus ring to the septal aspect to the aortic annulus plane (perpendicular to the atrioventricular plane) can be measured (Figure 7C).
Left ventricular long-axis diameter. A: Distance from apex to the geometric center of mitral valve annulus. B: Distance from the left atrial roof to the center of the mitral valve annulus. C: Relationship between left atrial appendage ostia and the mitral valve annulus: left atrial appendage ostia and mitral valve annulus defined by blue curves.
Cross sections above and below mitral valve. A: 5-mm increments above and below mitral valve annulus. B: Showing x-section at 10mm below mitral valve annulus in the left ventricle with best fit diameter measurement. C: Distance from mitral annulus ring to the aortic annulus plane (perpendicular to the atrioventricular plane plane).
Interventional cardiology shares characteristics with other minimally invasive therapies, such as laparoscopic procedures, that make it suitable for simulator-based learning: it requires complex understanding of 3D anatomy from 2D displays and fine hand-eye coordination.16 Complications from improperly performed cardiac interventional procedures may have catastrophic results. Basic skills must be attained before proficiency can be achieved in more complex procedures. Several reports show that intervention success is related to the volume of procedures performed by an operator.17 In complex, novel and infrequently performed procedures, the traditional invasive procedural teaching using a master-apprentice model with intense one-on-one contact between the teacher and the trainee may be insufficient, with a potentially high risk of procedural complications.
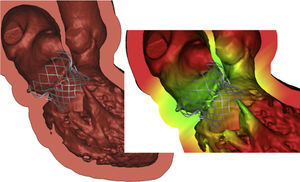
Starting from preoperative multislice computed tomography images and using the Mimics© Innovation Suite, the mitral valvar complex, including the calcifications, can be segmented and reconstructed in 3D. A 3D computer-aided design model of a transcatheter MV device is imported to Mimics© and positioned within the patients reconstructed anatomy (Figure 8). The assembly can then be exported to a finite-element solver to run simulations. With this prosthesis model, advanced, non-linear finite element simulations can be used to make several predictions: stent frame deformation, incomplete frame apposition, calcium movement and more. This allows for assessment of different access sites; antegrade access to the MV through either a direct transatrial approach, or via the venous system with transseptal access into the left atrium and transapical approach via a left-sided, mini-thoracotomy. We can test which of theses accesses would achieve more coaxial alignment of the valve annulus to the mitral prosthesis for proper valve deployment. Finally, we can assess the risk of complications, such as LVOT obstruction, papillary muscle interaction, valve regurgitation, prior to performing the intervention (Figure 8).
PHYSICAL PATIENT-SPECIFIC 3D HEART MODELS OF THE MITRAL VALVAR COMPLEX: 3D-PRINTING TECHNOLOGYThree-dimensional printing is a manufacturing technique used in engineering to generate prototype models using an additive layer-by-layer process. By transferring this technique to medicine, a 3D model of the human anatomy can be derived from patient imaging and 3D printed as a physical object. This process is enabled by segmenting the imaging data and converting it to 3D computerized surface models. These are then manufactured via a 3D printing technology to produce physical models. Three-dimensional printing enables the creation of life-like replicas of the human anatomy from various medical imaging modalities, such as computed tomography, magnetic resonance imaging and 3D echocardiography.18,19 As additive manufacturing, or 3D printing, has become more practical and affordable, a number of applications for the technology in the field of minimally-invasive cardiac interventions, and transcatheter heart intervention procedures in particular, have been considered.18–23 One promising area is percuntaneous heart valve replacement simulation.
Simulated MV complex created through this process with Mimics© (Materialise NV) may have significant potential for benefit in preoperative simulation and patient-specific planning for TMVR. In addition, it may serve as a method to enhance understanding of the complex anatomy of the MV.
SUMMARY AND FUTURE PROSPECTSNovel percutaneous transcatheter and minimally-invasive valve technologies for treating MR are rapidly emerging, but their optimal application remains to be elucidated. Percutaneous MV replacement is a promising technique in the experimental stages of human application with several anatomical and technical challenges that need to be overcome before widespread adoption.9–12
A better understanding of mitral anatomy-pathophysiology-device interaction is of great interest and requires cardiac imaging technology. Imaging is an integral part of transcatheter structural interventions and plays an important role in patient selection (screening), preprocedural planning, and procedural decision-making.22 In addition, the clinical applications of 3D modeling and 3D printing in structural heart disease are widespread. In 3 decades, 3D printing technology has grown from a niche manufacturing process to a $2.7-billion industry, responsible for the fabrication of a multitude of products including toys, wristwatches, airplane parts, food, and more. The application of this technology to medicine may accelerate equally dramatic change.
In the context of percutaneous mitral valve replacement, the creation of patient-specific 3D models and 3D printed physical models may provide new opportunities at various levels:
- 1.
From an interventional cardiologist perspective, it may enable: a) better understanding of the interrelationship of anatomical structures of the heart; b) detailed anatomic and geometric information on the MV complex; c) planning of technical strategies with virtual devices; and d) appreciation of potential procedural difficulties and assessment of the likelihood of success or failure for TMVR.
- 2.
From the manufacturer perspective, patient-specific physical models may be of particular value in the preclinical development of new devices by accurately portraying spatial relationships and device/anatomy fitting in a spectrum of diseased hearts from patients who could be potential candidates for TMVR.
- 3.
From an educational perspective, by enhancing the anatomic and pathological understanding of mitral valve disease, physical models can enrich patients’, students’, and physicians’ understanding of structural mitral valve disease and help to clarify the aims and limitations of percutaneous mitral valve replacement.
3D-heart models and bioprinting technology are just starting to become widely available at a more affordable cost. Thus, we expect that there will be rapid adoption of 3D printed models in medicine and minimally-invasive valve therapies. Despite these potential advantages, clinical studies are warranted to assess their role in the area of percutaneous heart procedures.
CONFLICTS OF INTERESTNone declared.