Cardiac device-related infections (CDRI) may be life-threatening and require early and accurate diagnosis. The aims of this study were to analyze the performance of positron emission tomography-computed tomography (PET/CT) in suspected CDRI, to assess changes to the initial diagnosis, and to identify a clinical subgroup deriving the greatest benefit from this imaging modality.
MethodsRetrospective study including patients evaluated by PET/CT for suspected CDRI from 2011 to 2018. We assessed PET/CT performance and the agreement between the initial, post-PET and definitive diagnoses. We also assessed changes in the diagnosis, depending on initial clinical suspicion, to identify patients deriving the greatest benefit from PET/CT.
ResultsWe included 44 patients. The prevalence of endocarditis was 57%. The sensitivity and specificity of PET/CT for the diagnosis of infective endocarditis were 0.84 and 0.95, respectively. Post-PET diagnosis improved the initial diagnosis by 45%. PET/CT correctly reclassified 57% of patients with initial suspicion of generator pocket infection by detecting lead infection.
ConclusionsPET/CT showed high diagnostic performance in suspected of CDRI and significantly improved the conventional diagnostic approach, especially in patients with initial suspicion of focal infection.
Keywords
According to Spanish pacemaker and defibrillator registries,1,2 the uses and indications of cardiac implantable electronic devices (CIED) are increasing, as well as recipients’ mean age and comorbidities. These aspects have been linked to a higher incidence of cardiac device-related infections (CDRI), estimated at 0.5% to 2.2%.3 CDRIs range from superficial generator pocket infection to infective endocarditis (IE) with or without embolisms. Underestimating a CDRI or delaying its treatment can trigger adverse effects ranging from CIED malfunction to patient death. However, its overestimation based on nonspecific symptoms can lead to unnecessary extractions, increased morbidity and mortality, and inadequate resource management.4
The diagnosis of IE is based on modified Duke criteria.5 Specific adaptations to these criteria for CIEDs have been proposed, such as the inclusion of clinical evidence of generator pocket infection or echocardiographic findings of lead vegetations as major criteria.6 Even so, their sensitivity in CIED patients is lower than in other settings (52%-70%).4 Echocardiography is the imaging technique of choice, but its use in these patients has limitations, such as acoustic shadow artifacts produced by the CIED components,4 lead movement, and similarities between thrombi or fibrin strands and true vegetations. Echocardiography provides uncertain results in up to 15% to 30% of cases.7 Currently, negative echocardiography does not allow us to rule out IE in patients with CIEDs.8
The search for improvements in the diagnosis of CDRI has generated growing interest in positron emission tomography-computed tomography (PET/CT) with 18F-fluorodeoxyglucose (18F-FDG-PET/CT). The European Society of Cardiology guidelines include abnormal FDG uptake on PET/CT as a major Duke criterion for prosthetic valve endocarditis. However, in CIED patients, its use is limited to suspected IE with positive blood cultures and negative echocardiography.8
The main objective of this study was to analyze the diagnostic performance of PET/CT in suspected CDRI in clinical practice conditions, which were not strictly limited to the guideline recommendations. Secondary objectives were to assess changes to the initial diagnosis and to identify a clinical subgroup deriving the greatest benefit from this imaging modality.
METHODSDesign and patientsA retrospective analysis was conducted of patients evaluated by PET/CT for suspected CDRI from August 2011 to January 2018. Diagnosis had been previously established according to local and systemic signs or symptoms of infection, microbiological findings (blood cultures or initial generator pocket exudate culture), and echocardiographic (transthoracic and transesophageal) findings.
Patients were classified by applying the diagnostic categories proposed by Sandoe et al.12 (table 1) at 3 time points: according to conventional diagnostic tests (pre-PET diagnosis), after including the results of PET/CT (post-PET diagnosis), and according to the reference standard (definitive diagnosis). We added 2 more categories, fever of unknown origin (FUO) and bacteremia without microbiological criteria of endocarditis according to modified Duke criteria (non-Duke bacteremia), because these categories constituted a reason for PET/CT for suspected CDRI in our setting. For post-PET diagnostic classification, in the event of a mismatch between echocardiography and PET/CT, the results of the latter technique were accepted. The consensus decision of the multidisciplinary endocarditis team was considered as the reference standard after a review of all the results, including those of the exudate culture and the follow-up data obtained at consultations 10 to 14 days after discharge or at the end of antibiotic therapy, with new standard blood cultures and surveillance of signs of infection. In addition, the team also included information obtained from a review of medical records over the first year in search of consultations or readmissions due to suspected infection.
Types of infection associated with intracardiac devices according to the definitions proposed by Sandoe et al.12
| Type of infection | Symptoms or signs |
|---|---|
| Inflammation after early implant | Erythema affecting the generator pocket incision site. No purulent exudate, dehiscence, or systemic symptoms of infection |
| Uncomplicated generator pocket infection | One of the following signs or symptoms present:• Cellulitus spreading to the generator pocket site• Purulent exudate (area > 1 cm) affecting the generator pocket incision site• Wound dehiscence• Erosion through skin with exposure of the generator or leads• Abscess or fistula in the generator pocket area, without systemic symptoms or signs of infection, and with negative blood cultures |
| Complicated generator pocket infection | The same as uncomplicated generator pocket infection but with evidence of at least one the following:• Lead or endocardial surface involvement• Systemic signs or symptoms of infection• Positive blood cultures |
| CIED lead infection | Symptoms or systemic signs of infection without signs of generator pocket infection |
| Definitive lead infectionEstablished under the following 2 conditions: | • Echocardiography consistent with vegetations attached to leads and presence of modified Duke microbiological criteria• Culture, histology, or molecular evidence of infection on explanted lead |
| Possible lead infectionEstablished under the following 2 conditions: | • Echocardiography consistent with vegetations attached to leads without presence of modified Duke microbiological criteria• Presence of modified major Duke microbiological criteria but no echocardiographic evidence of lead vegetations |
| CIED-related infectious endocarditis | Duke criteria for definite endocarditis satisfied, with echocardiographic evidence of valve involvement in patients with a CIED in situ |
CIED, cardiac implantable electronic device.
The microbiological samples were incubated for 14 days and the extracted CIED components underwent sonication. The study was approved by the Research Ethics Committee.
Positron emission tomography-computed tomographyThe PET/CT acquisition protocols were followed in accordance with the guideline recommendations.9–11 We applied a protocol for the suppression of the myocardial physiological uptake of 18F-FDG, which consisted of prolonged fasting (16hours) and, since 2016, an intravenous bolus (i.v.) of sodium heparin (50 IU/kg) 15minutes before injection of the radiotracer. After confirming that blood glucose levels were less than or equal to 200mg/dL, we administered a standard dose of 18F-FDG 370 MBq i.v. After 60minutes, images were acquired from the cranial vertex to the upper third of the thighs using a Biograph 6 PET/CT device (Siemens, Germany).
The images underwent qualitative analysis using Leonardo workstations with Syngo 2002A_R1.0 imaging software and Syngo.via (Siemans, Germany). Focal or heterogeneous uptake superior to the vascular pool or healthy tissue adjacent to the generator pocket (local infection), leads, or prosthetic valve (IE) were considered suggestive of infection. We also evaluated findings compatible with septic embolism, infectious/inflammatory disease unrelated to CIEDs, or tumora disease.
Variables and statistical analysisFor diagnostic performance analysis, we used reference standard contingency tables to calculate the sensitivity, specificity, positive and negative predictive values, and positive and negative odds ratios of echocardiography and PET/CT.
To be able to construct the contingency tables, the definitive diagnosis categories agreed upon as the reference standard were as follows:
- •
No IE: superficial inflammation, uncomplicated generator pocket infection, FUO, and non-Duke bacteremia.
- •
IE: complicated generator pocket infection, possible or definitive lead infection, and definitive CIED-IE/prosthetic valve infection.
The following groups were established for the imaging tests:
- •
IE: mobile echogenic mass (echocardiography) or pathological uptake (PET/CT) in leads or endocardial surface.
- •
No IE: due to absence of vegetation (echocardiography) or lack of uptake or uptake limited to the generator pocket or unrelated to CIED (PET/CT).
Uncertain results on imaging tests were considered positive, favoring S, due to the life-threatening nature of the process under study. A second analysis was conducted in which uncertain results were considered negative, while taking into account that this approach would be less acceptable in clinical practice.
PET/CT performance was calculated based on the assessment of isolated generator pocket infection or deep generator pocket infection, and the following groups were established:
- •
Generator pocket infection: uncomplicated and complicated generator pocket infection.
- •
Nongenerator pocket infection: superficial inflammation, possible or definitive lead infection, definitive CIED-IE/prosthetic valve infection, FUO, and non-Duke bacteremia.
The weighted kappa index was used to calculate the agreement between pre-PET diagnosis and definitive diagnosis, and between post-PET diagnosis and definitive diagnosis in relation to the distribution of patients in the diagnostic categories. To this end, the categories of possible or definitive lead infection and definitive CIED-IE/prosthetic valve infection were grouped as “CIED-IE/prosthetic valve infection” and the categories of FUO and non-Duke bacteremia were grouped as “no CDRI”. Applying this grouping, the percentage of correct diagnostic change within each pre-PET diagnostic category was analyzed after including the PET/CT results to identify a clinical subgroup deriving the greatest benefit from this imaging modality.
We used the SPSS (Version 22.0) statistical software package and calculators to evaluate diagnostic tests conducted by the Ramón y Cajal Hospital Research Unit (Madrid, Spain).13
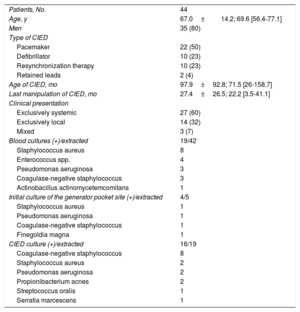
RESULTSPatient characteristicsWe included 44 patients. Table 2 shows their main characteristics. Most CIEDs (79%) had at least 2 leads. All generators were implanted in the left anterior thoracic wall. The initial culture of the pocket region was taken if there was lesion exudate. In this case, the swab sample was taken directly from the wound.
Patient characteristics
| Patients, No. | 44 |
| Age, y | 67.0±14.2; 69.6 [56.4-77.1] |
| Men | 35 (80) |
| Type of CIED | |
| Pacemaker | 22 (50) |
| Defibrillator | 10 (23) |
| Resynchronization therapy | 10 (23) |
| Retained leads | 2 (4) |
| Age of CIED, mo | 97.9±92.8; 71.5 [26-158.7] |
| Last manipulation of CIED, mo | 27.4±26.5; 22.2 [3.5-41.1] |
| Clinical presentation | |
| Exclusively systemic | 27 (60) |
| Exclusively local | 14 (32) |
| Mixed | 3 (7) |
| Blood cultures (+)/extracted | 19/42 |
| Staphylococcus aureus | 8 |
| Enterococcus spp. | 4 |
| Pseudomonas aeruginosa | 3 |
| Coagulase-negative staphylococcus | 3 |
| Actinobacillus actinomycetemcomitans | 1 |
| Initial culture of the generator pocket site (+)/extracted | 4/5 |
| Staphylococcus aureus | 1 |
| Pseudomonas aeruginosa | 1 |
| Coagulase-negative staphylococcus | 1 |
| Finegoldia magna | 1 |
| CIED culture (+)/extracted | 16/19 |
| Coagulase-negative staphylococcus | 8 |
| Staphylococcus aureus | 2 |
| Pseudomonas aeruginosa | 2 |
| Propionibacterium acnes | 2 |
| Streptococcus oralis | 1 |
| Serratia marcescens | 1 |
(+), positive result, with growth of microorganisms; CIED, cardiac electronic implantable device.
Values are expressed as No. (%), mean±standard deviation, or median [interquartile range].
Table 3 shows the tests that were performed and patient classification according to the results. Table 4 shows details of blood cultures according to the definitive diagnosis.
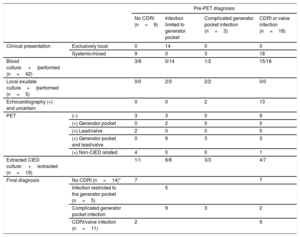
Initial and definitive diagnostic classification of patients and test results
| Pre-PET diagnosis | |||||
|---|---|---|---|---|---|
| No CDRI (n=9) | Infection limited to generator pocket | Complicated generator pocket infection (n=3) | CDRI or valve infection (n=18) | ||
| Clinical presentation | Exclusively local | 0 | 14 | 0 | 0 |
| Systemic/mixed | 9 | 0 | 3 | 18 | |
| Blood culture:+/performed (n=42) | 3/8 | 0/14 | 1/2 | 15/18 | |
| Local exudate culture+/performed (n=5) | 0/0 | 2/3 | 2/2 | 0/0 | |
| Echocardiography (+) and uncertain | 0 | 0 | 2 | 13 | |
| PET | (–) | 3 | 3 | 0 | 9 |
| (+) Generator pocket | 0 | 2 | 0 | 0 | |
| (+) Lead/valve | 2 | 0 | 0 | 5 | |
| (+) Generator pocket and lead/valve | 0 | 9 | 3 | 3 | |
| (+) Non-CIED related | 4 | 0 | 0 | 1 | |
| Extracted CIED culture:+/extracted (n=19) | 1/1 | 8/8 | 3/3 | 4/7 | |
| Final diagnosis | No CDRI (n=14)* | 7 | 7 | ||
| Infection restricted to the generator pocket (n=5) | 5 | ||||
| Complicated generator pocket infection | 9 | 3 | 2 | ||
| CDRI/valve infection (n=11) | 2 | 9 | |||
(+), positive result; (–), negative result; CIED, cardiac implantable electronic device; CDRI, cardiac device-related infection; No CDRI, includes categories of fever without a focus and non-Duke bacteremia; PET, positron emission tomography.
Complicated generator pocket infection is defined as generator pocket and lead infection. CDRI/valve infection includes the categories of possible or definitive lead infection, CDRI, or valvular infective endocarditis.
Bacteremia unrelated to Enterococcus faecalis (n=3) and Actinobacillus actinomycetemcomitans (n=1), catheter-related bacteremia due to S. aureus (n=3), pulmonary infectious focus (n=2), active pneumonitis (n=1), pericarditis (n=1), tumor-related fever (n=1), suspected cholangitis (n=1), and fever of unknown origin (n=1).
Distribution of blood culture results according to the definitive diagnosis
| Final diagnosis | Blood cultures extracteda | Blood cultures (+) | Blood cultures (–) with/without antibiotic therapy |
|---|---|---|---|
| No CDRI (n=14) | 13 | 7 (54%)b | 2/4 |
| Uncomplicated generator pocket infection | 5 | 0 | 1/4 |
| Complicated generator pocket infection or deep infection (n=25) | 24 | 12 (50%) | 1/11 |
CDRI, cardiac device-related infection.
3 baseline extractions were collected into 2 bottles (1 aerobic, 1 anaerobic), separated by a maximum of 1 hour. If the result was positive, serial determinations were made every 24 to 48hours until 2 consecutive negative results were obtained.
In the 7 cases corresponding to bacteremia (pathogens specified in table 3), all blood cultures showed negative results 48hours after the start of antibiotic treatment.
In 25 patients (57%), IE was established according to the reference standard, which was higher among patients referred with local infection (64% vs 53%).
Table 2 and table 3 show the results of CIED extraction and culture in 19 patients (43%). CIED extraction was more common among patients with a definitive diagnosis of IE than among those with a definitive diagnosis of uncomplicated generator pocket infection or no CDRI (61.5% vs 10.5%; P = .01).
Treatment was conservative in 25 patients (57%): of these, 7 had a definitive diagnosis of IE and high surgical risk and were treated with antibiotic therapy i.v. for 4 to 6 weeks; 4 were treated with chronic oral antibiotic therapy and antibiotic therapy i.v. for 6 weeks; and 3 had prosthetic valve IE unrelated to the CIED. During follow-up, infection was controlled in 24 patients: however, 1 patient died at 8 months due to sepsis of intestinal origin. The eighth patient with a definitive diagnosis of complicated infection was initially treated as having uncomplicated infection (oral antibiotic therapy for 4 weeks). Unfavorable clinical course led to CIED extraction some months later.
The 5 patients with a definitive diagnosis of generator pocket infection were treated with oral antibiotic therapy for 4 weeks, without relapse of infection during follow-up. CDRI was ruled out in 14 patients (32%) and a specific alternative diagnosis was reached in 9 of them (table 3).
Diagnostic performance for the detection of infective endocarditisTable 3 shows the findings of echocardiography and PET/CT. All patients underwent at least 1 echocardiography (34 transesophageal, 34 transthoracic, and 25 both). In case of disagreement, the result of the transesophageal approach was accepted. Echocardiographic examination was considered positive in 9 patients (20%), uncertain in 6 (14%), and negative in the other patients.
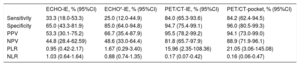
The results of PET/CT and echocardiography were concordant in 19 patients (43%): 13 patients without findings and 6 patients with findings of IE. Table 5 shows the diagnostic performance of PET/CT and echocardiography. The sensitivity of PET/CT was significantly higher than that of echocardiography, and showed pathological uptake in 15 of the 16 false negatives (FN) on echocardiography. Uptake was located in the external course of the leads in 14 patients and in the aortic valve in 1 patient.
Diagnostic performance of ultrasound and PET/CT in the detection of infective endocarditis and of PET/CT in generator pocket infection
| ECHO-IE, % (95%CI) | ECHO*-IE, % (95%CI) | PET/CT-IE, % (95%CI) | PET/CT-pocket, % (95%CI) | |
|---|---|---|---|---|
| Sensitivity | 33.3 (18.0-53.3) | 25.0 (12.0-44.9) | 84.0 (65.3-93.6) | 84.2 (62.4-94.5) |
| Specificity | 65.0 (43.3-81.9) | 85.0 (64.0-94.8) | 94.7 (75.4-99.1) | 96.0 (80.5-99.3) |
| PPV | 53.3 (30.1-75.2) | 66.7 (35.4-87.9) | 95.5 (78.2-99.2) | 94.1 (73.0-99.0) |
| NPV | 44.8 (28.4-62.59) | 48.6 (33.0-64.4) | 81.8 (65.7-97.9) | 88.9 (71.9-96.1) |
| PLR | 0.95 (0.42-2.17) | 1.67 (0.29-3.40) | 15.96 (2.35-108.36) | 21.05 (3.06-145.08) |
| NLR | 1.03 (0.64-1.64) | 0.88 (0.74-1.35) | 0.17 (0.07-0.42) | 0.16 (0.06-0.47) |
95%CI, 95% confidence interval calculated using the Wilson method; ECHO, echocardiography; ECHO-IE, echocardiography performance in the diagnosis of infective endocarditis; NLR, negative likelihood ratio; NPV, negative predictive value; PET/CT, positron emission tomography-computed tomography; PET/CT-IE, PET/CT performance in the diagnosis of infective endocarditis; PET/CT-pocket, PET/CT performance in the diagnosis of generator pocket infection; PLR, positive likelihood ratio; PPV, positive predictive value.
The specificity of PET/CT was higher than that of echocardiography without reaching statistical significance. None of the 7 patients with false positive (FP) findings on echocardiography showed pathological uptake. The results of PET/CT are supported by the finding that the disease was resolved after conservative treatment not directed at IE in 5 patients and the absence of infection during and after CIED extraction in the other 2 patients.
PET/CT showed 1 FP result for IE consisting of pathological uptake in a proximal lead of a bicameral CIED that had been replaced 35 days earlier. Oral antibiotic therapy for 1 month was administered for local infection and negative echocardiogram. After treatment, PET/CT showed persistent uptake, although of decreased intensity. However, there were no new infectious episodes during the year of follow-up, and the definitive diagnosis was uncomplicated CIED infection.
Four FNs on PET/CT were detected, 3 in patients with systemic infection, positive findings on echocardiography, and positive blood cultures. Because of the risk of CIED extraction, they were treated with antibiotic therapy i.v. for 6 weeks. The team established a definitive diagnosis of suspected lead infection in 2 of these patients. Due to severe heart failure, the third patient received a transplant after initial antibiotic therapy; pretransplant echocardiography showed an image compatible with treated vegetation. The fourth patient showed local infection and negative findings on echocardiography; no blood cultures were taken. Superficial infection was suspected and oral antibiotic therapy was initiated. An unfavorable response led to CIED extraction; Staphylococcus epidermidis infection was confirmed by generator and lead culture. Myocardial suppression was optimal in 3 patients and suboptimal in 1. Antibiotic therapy time prior to PET/CT was 0, 1, 4, and 8 days, respectively.
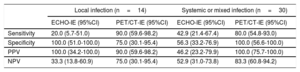
Table 6 shows the diagnostic performance of echocardiography and PET/CT according to clinical presentation. The sensitivity of PET/CT was particularly high in patients with local symptoms. In patients with systemic symptoms, a correlation was found between pathological uptake in the deep components of the CIED or in the endocardial surface and the definitive diagnosis of IE.
Performance of imaging tests for the diagnosis of ie according to initial clinical suspicion
| Local infection (n=14) | Systemic or mixed infection (n=30) | |||
|---|---|---|---|---|
| ECHO-IE (95%CI) | PET/CT-IE (95%CI) | ECHO-IE (95%CI) | PET/CT-IE (95%CI) | |
| Sensitivity | 20.0 (5.7-51.0) | 90.0 (59.6-98.2) | 42.9 (21.4-67.4) | 80.0 (54.8-93.0) |
| Specificity | 100.0 (51.0-100.0) | 75.0 (30.1-95.4) | 56.3 (33.2-76.9) | 100.0 (56.6-100.0) |
| PPV | 100.0 (34.2-100.0) | 90.0 (59.6-98.2) | 46.2 (23.2-79.9) | 100.0 (75.7-100.0) |
| NPV | 33.3 (13.8-60.9) | 75.0 (30.1-95.4) | 52.9 (31.0-73.8) | 83.3 (60.8-94.2) |
95%CI, 95% confidence interval calculated using the Wilson method; ECHO-IE, echocardiography performance in the diagnosis of infective endocarditis; NPV, negative predictive value; PET/CT-IE, PET/CT performance in the diagnosis of infective endocarditis; PPV, positive predictive value.
Table 3 and table 5 show the performance of PET/CT in generator pocket infection. The only FP was associated with a patient with systemic infection undergoing cardiac transplant due to hypertrophic cardiomyopathy with incomplete CIED removal 3 months earlier. Because of intermittent bacteremia due to Pseudomonas aeruginosa, PET/CT was performed showing uptake in the lead retained in the vena cava and aortic root. P. aeruginosa infection was confirmed by aortic root and lead culture following aortic root replacement and lead extraction. PET/CT also showed mild uptake in the generator pocket site. Infection was finally attributed to postoperative inflammation in the absence of other clinical or microbiological data confirming local infection.
Two of the 3 patients with FN on PET/CT had local infection. Treatment was conservative with an unfavorable clinical course. The CIEDs were extracted and culture performed, which confirmed uncomplicated and complicated CDRI, respectively. The third patient with FN on PET/CT had local infection and heart failure. After 2 weeks of oral antibiotic therapy with local improvement, the definitive diagnosis was assumed to be uncomplicated generator pocket infection. None of the patients had been started on antibiotic therapy before the PET/CT study.
Improvement in diagnostic classificationTable 7 shows the distribution of patients according to the 3 diagnostic steps. Concordance between the post-PET diagnosis and the definitive diagnosis was excellent (κ=0.81) and concordance between the pre-PET diagnosis and definitive diagnosis was low (κ=0.36)
Distribution of patients according to the time of diagnosis
| Pre-PET diagnosis, No. (%) | Post-PET diagnosis, No. (%) | Final diagnosis, No. (%) | |
|---|---|---|---|
| Absence of CDRI (FUO, non-Duke bacteremia) | 9 (20) | 15 (34) | 14 (32) |
| Infection limited to generator pocket | 14 (32) | 5 (11) | 5 (11) |
| Complicated generator pocket infection | 3 (7) | 14 (32) | 14 (32) |
| CIED-IE/valve infection (suspected or definitive lead infection and CIED-IE) | 18 (41) | 10 (23) | 11 (25) |
CDRI, cardiac device-related infection; CIED-IE, CIED-related IE; FUO, fever of unknown origin, No., number of patients; PET, positron emission tomography.
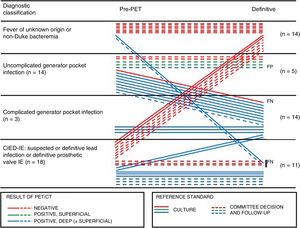
Using the model of Graziosi et al.,14figure 1 shows individual changes between the initial and final diagnostic classification according to the results of PET/CT and the reference standard. The most promising PET/CT results were obtained in patients who had been initially referred for uncomplicated generator pocket infection, because 8 of 14 patients (57%) were correctly reclassified by the detection of pathological uptake in leads, indicating complicated infection. In 7 of 21 patients (33%) with suspected deep infection, the absence of pathological uptake contributed to its correct exclusion. Among the 9 patients referred for FUO or non-Duke bacteremia, PET/CT detected deep infection in 2 patients and increased the level of certainty in ruling out CDRI and provided alternative diagnoses in 4 patients.
Individual changes between the initial and final diagnostic classification according to the results of PET/CT. CIED-IE: infective endocarditis associated with an intracardiac device; IE: infective endocarditis; FN: false negative; FP: false positive; PET/CT, positron emission tomography-computed tomography.
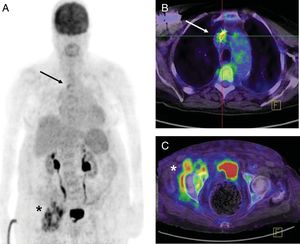
In 6 patients (14%), PET/CT showed uptake in the deep components of the CIED as well as pulmonary uptake indicative of embolic origin. In 2 patients (4%) with lead infection and bacteremia, the technique detected concomitant osteoarticular infection (figure 2). In a previously described patient, it detected an unsuspected aortic root infection.
Pacemaker patient, 84 years, last manipulation 3 years earlier. PET/CT was performed for fever and methicillin-sensitive Staphylococcus aureus bacteremia. A: volumetric imaging. B: lead uptake in upper cava according to PET/CT fusion imaging (arrow). C: an unsuspected septic focus on the right hip with periarticular fluid collections (asterisk). Considering the baseline situation, conservative treatment was administered consisting of cefazolin i.v. and oral rifampicin for 6 weeks. Blood cultures were negative on day 5. This treatment was followed by oral cephalosporin indefinitely without new episodes of infectious exacerbation during the subsequent year. PET/CT, positron emission tomography-computed tomography.
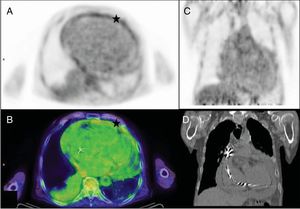
In 4 of the 14 patients (29%) in whom the definitive diagnosis ruled out CDRI, PET/CT facilitated an alternative diagnosis by the detection of interstitial pneumonitis, active pneumonia, papillary thyroid carcinoma, and pericarditis (figure 3).
Patient, 76 years, with implantable automatic defibrillator, last manipulation 3 years earlier. PET/CT performed for general malaise, fever of unknown origin, and negative blood cultures showing pericardial pathological uptake. A: PET transaxial image. B: PET/CT transaxial image (asterisk). C and D: absence of lead uptake (coronal images). A diagnosis of pericarditis was made. Colchicine treatment was initiated, with clinical remission. PET/CT, positron emission tomography-computed tomography.
In 4 patients (9%) intestinal uptake was detected and confirmed as low-grade tubular adenoma in 2 patients and high-grade neoplasia in 1 patient. Complementary studies could not be performed in the fourth patient due to early death.
DISCUSSIONPET/CT showed high diagnostic performance in suspected CDRI and significantly improved the conventional diagnostic approach. Its performance was particularly notable in patients with suspected local infection. In addition, negative findings on PET/CT or findings that indicated another disease on PET/CT increased the level of certainty in ruling out IE.
Suspected infection in patients with CIEDs is a challenge that requires the use of clinical, microbiologic, and echocardiographic criteria. In our series, the percentage of IE was higher in patients with suspected local infection, which shows the low specificity of the clinical picture alone. Blood cultures were negative in 12 (48%) of the 25 patients with a final diagnosis of IE. Although this finding is in line with previously published figures, which estimate that blood cultures will be negative in 25% to 70% of patients with CIED-IE,15 it should be noted that 75% of the negative results were obtained in patients with a pre-PET diagnosis of uncomplicated infection. Echocardiography is the first-line imaging technique in cases of suspected CDRI. In this study, PET/CT showed high sensitivity and specificity and helped resolve inconclusive findings on echocardiography. The sensitivity values of echocardiography were lower than those reported in a meta-analysis (82%-96%).16 The difference was possibly due to multiple factors. First, we only analyzed the echocardiographic results of patients subsequently assessed by PET/CT. Patients with unambiguous pathological ultrasound findings were probably not included. The extracameral location of uptake and the ability of PET/CT to identify CDRI early may also have had an effect.4 On the other hand, the echocardiographic FPs should be interpreted with caution because, in most of these cases, the treatment was conservative, which leads to greater uncertainty in the final diagnosis.
We qualitatively analyzed the PET/CT images and obtained diagnostic performance values similar to those of a recent meta-analysis. It follows that the sensitivity of studies that use qualitative analysis (87% [64%-100%]) is similar to that obtained by those that use semiquantitative criteria (85% [65%-95%]). Specificity is higher under semiquantitative criteria (97% [80-100%] vs 90% [86%-100%]).16 Although it appears that intense uptake would tend to indicate infection,17 current evidence does not allow a threshold to be established that would discriminate inflammation from infection. This situation is partly due to the current variety of protocols and criteria for analyzing images.18 More standardized studies are needed for one type of assessment approach to be recommended over another.
The literature reports an FN rate on PET/CT of 0% to 43%.16 Lower sensitivity results, such as those reported by Cautela et al.,19 are related to more patients being on antibiotic therapy at the time of the study. In their series, 89% of patients with FNs were under treatment before PET/CT. In contrast, Amraoui et al.20 reported that 14 out of 16 FN patients and 13 out of 19 true positive patients were receiving antibiotic therapy without significant differences between groups (P=.24). We included 36 patients (82%) under treatment and only 3 (8%) were FN. Until further results are available, it is recommended that PET/CT is performed as soon as possible.18
A meta-analysis found that FPs on PET/CT ranged between 0% and 8%.16 FP findings can occur from postsurgical inflammation, without a clear time margin, although it seems that the risk of FPs in CIED of more than 6 months duration is very low.17 The only FP finding in our study was related to a recently replaced CIED.
The absence of appropriate patient preparation to ensure myocardial suppression of physiologic uptake can lower the accuracy of PET/CT and can lead to FPs and FNs.16 The protocols used vary between series; prolonged fasting (4-12hours), heparin administration, a high fat diet, and combined protocols have been described.18 We achieved complete suppression in 31 patients (70%) using a methodology similar to that used by of Pizzi et al.21 In their series of 92 patients, complete suppression was achieved in 53% and partial suppression in 23%.
The broad spectrum of patients included in this study has demonstrated the effectiveness of PET/CT to detect deep infection in patients with an initial suspicion of local infection. Previous series have shown positive lead cultures in 50% to 72% of the extractions performed in patients with local infection.22,23 There were findings on PET/CT of deep infection in 57% of patients with initial suspicion of local infection. In the group of patients with a low suspicion of CDRI (non-Duke bacteremia or FUO), PET/CT detected deep infection in 2 patients (22%). Ploux et al.24 found even higher positive lead cultures (60%) in patients with low suspicion, FUO, negative blood culture, and negative findings on echocardiography, thus supporting the utility of PET/CT in this subgroup.
Among the 14 patients whose definitive diagnosis ruled out any form of CDRI, blood cultures and echocardiography were positive in 7 (50%) and 6 (43%), respectively. In all patients, PET/CT findings in relation to the CIEDs were negative.
Amraoui et al.20 recently analyzed the ability of PET/CT to detect embolisms, other foci of infectious origin, or unsuspected cancer. They found that PET/CT detected embolisms and cancers in 29% and 9% of their patients, respectively. The authors highlight the relevance of these findings for designing an appropriate therapeutic strategy. In their case, it led to an overall prolongation of antibiotic therapy. In our series, findings of embolism or infection not associated with CIEDs in approximately 30% of patients and of possible neoplastic disease in approximately 11% of patients reduced diagnostic uncertainty by increasing the certainty of deep infection or by providing alternative diagnoses.
LimitationsThis study has several limitations. First, its main limitation is its retrospective design, which makes it difficult to identify and control confounding factors, thus limiting the extrapolation of the results. Second, selection bias may be present, because patients with clear surgical indications or in a severe or unstable clinical situation may not have been referred to PET/CT. Such bias would most likely affect the results of echocardiography in our series, which therefore would not reflect actual echocardiographic performance in patients with CIEDs. In our study, a broad spectrum of patients with systemic infection were referred to PET/CT and therefore IE was one of the more likely diagnostic outcomes. In contrast, patients with clear generator pocket infection are more likely to have infection of the deep components of the CIED. This would explain why, in our series, the percentage of IE was higher in patients referred for local symptoms. Finally, PET/CT images were analyzed when the results of the other tests were already known. Likewise, the endocarditis team established the definitive diagnosis after knowing the result of the PET/CT, which may have influenced their decisions. In both cases there may have been diagnostic bias.
CONCLUSIONSPET/CT showed high sensitivity and specificity in suspected CDRI, both in the assessment of local infection and of CIED-IE. The inclusion of PET/CT significantly improved the conventional diagnostic approach regarding the classification of patients with suspected CDRI. The greatest diagnostic impact of the use of PET/CT was observed in the group of patients with suspected local infection. These results should be validated in prospective studies including more patients.
CONFLICTS OF INTERESTNone declared.
- -
There has been an increase in the incidence of CDRIs, which are potentially fatal processes.
- -
Infections can affect different components of CIEDs, with or without hematogenous foci. It is crucial to determine the spread of infection in order to provide correct treatment.
- -
18F-FDG-PET/CT is a morphofunctional imaging technique that can rapidly detect infectious/inflammatory foci.
- -
Published studies on its use in suspected CDRI show satisfactory results, but its place in diagnostic algorithms has not been defined.
- -
This study shows the performance and utility of 18F-FDG-PET/CT in real-world clinical practice.
- -
The study shows that there is wide variability in symptoms as well as in the degree of suspicion of CDRI and its extent. Thus, indication for PET/CT should be flexible and based on communication between the services involved.
- -
Our practical approach has facilitated the identification of a subgroup of patients deriving the greatest diagnostic benefit: those with suspected local infection, which is an indication not included in the treatment guidelines for CDRI.