
Fatty acid metabolic dysregulation in mitochondria is a common mechanism involved in the development of heart failure (HF) and atrial fibrillation (AF). We evaluated the association between plasma acylcarnitine levels and the incidence of HF or AF, and whether the mediterranean diet (MedDiet) may attenuate the association between acylcarnitines and HF or AF risk.
MethodsTwo case-control studies nested within the Prevención con dieta mediterránea (PREDIMED) trial. High cardiovascular risk participants were recruited in Spain: 326 incident HF and 509 AF cases individually matched to 1 to 3 controls. Plasma acylcarnitines were measured with high-throughput liquid chromatography-tandem mass spectrometry. Conditional logistic regression models were fitted to estimate multivariable OR and 95%CI. Additive and multiplicative interactions were assessed by intervention group, obesity (body mass index ≥ 30 kg/m2), and type 2 diabetes.
ResultsElevated levels of medium- and long-chain acylcarnitines were associated with increased HF risk (adjusted ORperDE, 1.28; 95%CI, 1.09-1.51 and adjusted ORperDE, 1.21; 95%CI, 1.04-1.42, respectively). A significant association was observed for AF risk with long-chain acylcarnitines: 1.20 (1.06-1.36). Additive interaction of the association between long-chain acylcarnitines and AF by the MediDiet supplemented with extra virgin olive oil (P for additive interaction=.036) and by obesity (P=.022) was observed in an inverse and direct manner, respectively.
ConclusionsAmong individuals at high cardiovascular risk, elevated long-chain acylcarnitines were associated with a higher risk of incident HF and AF. An intervention with MedDiet+extra-virgin olive oil may reduce AF risk associated with long-chain acylcarnitines.
This trial was registered at controlled-trials.com (Identifier: ISRCTN35739639).
Keywords
Heart failure (HF) and atrial fibrillation (AF) have emerged as important cardiovascular (CV) diseases contributing to the increasing burden of chronic diseases and health care costs.1 Both HF and AF result from cumulative exposure of shared CV risk factors that promote common and concurrent biological pathways such as the release of inflammation mediators which are involved in cardiac structural and electrophysiological remodeling.2
By studying HF and AF simultaneously, we can acquire a better understanding of their common underlying mechanisms as well as the key pathophysiological differences between them.3 Fatty acid metabolic dysregulation in mitochondria has been proposed as a common mechanism involved in the development of HF and AF. Moreover, the accumulation of long-chain acylcarnitines (LCAC) in plasma and tissue play an active role in inflammation and insulin resistance.4
In the Prevención con dieta mediterránea (PREDIMED) study, the mediterranean diet (MedDiet) intervention mitigated the association of high acylcarnitine (AC) concentrations with the risk of major CV events (ie, myocardial infarction, stroke, or CV death) compared with the control group.5 However, similar evidence is not currently available for AF and HF. We designed 2 case-control studies nested within the PREDIMED trial to evaluate the associations between ACs and the incidence of HF or AF, and whether the MedDiet could mitigate the harmful effects associated with increased baseline AC levels. As a secondary aim, since both cardiovascular diseases share common risk factors,6 we assessed the association between ACs and a composite outcome including participants with either HF or AF.
METHODSPopulation and designWe designed 2 case-control studies nested within the PREDIMED trial.7 We selected 326 incident cases of HF and 509 AF cases after excluding prevalent cases and incident cases without available samples. Incidence density sampling with replacement was used as the control sampling method.8 Thus, controls were randomly selected from all participants at risk at the time of the incident case occurrence, and selected controls could be selected again as a control for another index case, and they could later become a case. Results from a simulation study considered incidence density sampling with replacement as the least biased and most efficient method for control sampling in nested case-control studies.9 Controls were matched by recruitment center, year of birth (± 5 years), and sex. We selected 1 to 3 matched controls per case. shows the flow chart of the participant selection process.
The protocol was approved by the research ethics committees at all study locations, and all participants provided written informed consent.
OutcomesHF and AF were a priori defined as secondary endpoints in the PREDIMED trial protocol. In this analysis, we included all HF and AF incident cases diagnosed from 2003 to December 2017. One center stopped follow-up in December 2014 and all participants from that center were censored at that date to be selected as controls.
Information on these outcomes was collected from continuous contact with participants and primary health care physicians, annual follow-up visits, yearly ad hoc reviews of medical charts and annual consultation of the National Death Index. This information was collected by physicians who were blinded to the intervention groups and metabolomics measurements. If a clinical diagnosis of HF or AF was found, all relevant documentation, including clinical records of hospital discharge, outpatient clinics and family physicians’ records were obtained. Medical charts were sent anonymously to the Clinical Endpoint Committee. This documentation was evaluated by 2 cardiologists separately and if they did not agree on the classification of the event, a third cardiologist (the chair of the committee) intervened. In some cases, more information was requested to complete the adjudication. The Endpoint Committee adjudicated the events according to criteria prespecified in a “Manual of operations” ().
Sample collection and metabolomic analysisDuring the baseline visit, ie, years before the development of AF or HF, participants provided blood samples after a fast of at least 8hours. All samples were processed at each recruiting center no later than 2hours after collection and were stored at−80°C until their analysis. Samples from matched case-control pairs were shipped and assayed in the same analytical run, varied at random to reduce bias and interassay variability. The metabolomics analysis is described at the . Information about the mass to charge ratio and retention time is shown in .
CovariatesBaseline questionnaires were used to collect sociodemographic, lifestyle variables, prevalent and family history of diseases, and medication use. Leisure-time physical activity was measured with the validated version of the Minnesota Leisure-Time Physical Activity questionnaire.10 Incident coronary heart disease (any diagnosis of angina, myocardial infarction, or coronary revascularization procedures) and stroke (any diagnosis of either ischemic or hemorrhagic stroke, and also transient ischemic attacks) were collected blindly to the metabolomics information, according to the diagnosis criteria applied by the Clinical Endpoint Committee.
Statistical analysisIndividual metabolite values were normalized and scaled to multiples of 1 standard deviation (SD) using the rank-based inverse normal transformation. The mean±SD was used to describe quantitative traits and absolute number and percentage to describe categorical variables.
We calculated the Pearson correlation coefficient for free carnitine, ACs and branched-chain amino acids. We also ran linear regression models using AC scores as dependent variables and CV risk factors as independent variables and adjusted for age and sex.
We fitted crude and multivariable conditional logistic regression models to account for the matching between cases and controls. We calculated matched odds ratios (OR) and their 95% confidence intervals (95%CI) for HF or AF in the comparisons of upper quartiles of the ACs vs the lowest quartile, and for each SD of ACs using them as continuous variables. Quartile cutoff points were calculated according to the distribution of ACs among controls (ie, participants without HF or AF). The multivariable model was adjusted for intervention group (MedDiet+extra virgin olive oil [EVOO], MedDiet+nuts or control), smoking status (never/current/former), body mass index (BMI) (kg/m2), leisure-time physical activity (metabolic equivalent task [MET]-min/d), prevalent chronic diseases (hypertension, type 2 diabetes [T2D] and dyslipidemia), and medication use, including angiotensin converting-enzyme inhibitors, diuretics, other antihypertensive treatments, statins and other lipid-lowering agents, insulin, oral hypoglycemic agents, and antiplatelet therapy. As an ancillary analysis, we additionally adjusted for incident coronary heart disease (angina, myocardial infarction, coronary revascularization procedures) and stroke (either ischemic or hemorrhagic, including transient ischemic attacks) diagnosed during the follow-up but before the diagnosis of HF or AF. Adjustment for multiple comparisons was based on the false discovery rate procedure as proposed by Simes.11 Additional statistical analyses are described in the .
Statistical analyses were performed using Stata/SE version 15.1 (Stata Corp).
RESULTSWe analyzed data from 2 case-control studies nested within the PREDIMED trial. The number of incident HF cases was 326 and that of incident AF cases was 509. In total, there were 727 cases because 108 participants developed both AF and HF during the follow-up (). Participants’ characteristics in both case-control studies nested within the PREDIMED trial are shown in table 1.
Baseline participant characteristics of HF and AF cases and controls
| Case-control set for HF | Case-control set for AF | |||
|---|---|---|---|---|
| Controlsa | HF cases | Controlsa | AF cases | |
| n | 426 | 326 | 617 | 509 |
| Age, y | 70.4±5.9 | 70.3±5.8 | 68.5±6.1 | 68.3±6.1 |
| Female sex, % | 54.2 | 58.3 | 49.3 | 49.7 |
| Intervention group, % | ||||
| MedDiet+EVOO | 37.6 | 30.1 | 36.5 | 31.4 |
| MedDiet+nuts | 26.5 | 32.5 | 28.5 | 31.4 |
| Control | 35.9 | 36.4 | 35.0 | 37.1 |
| Smoking, % | ||||
| Never | 61.3 | 59.8 | 58.0 | 58.7 |
| Former | 27.5 | 25.8 | 28.7 | 726.9 |
| Current | 11.2 | 14.4 | 13.3 | 14.4 |
| Physical activity, METs-min/d | 217±220 | 216±204 | 229±220 | 228±216 |
| Education, % | ||||
| Elementary or lower | 81.7 | 85.0 | 78.8 | 76.0 |
| Secondary or higher | 18.3 | 15.0 | 20.2 | 24.0 |
| Total energy intake, kcal/d | 2279±637 | 2217±632 | 2342±603 | 2285±600 |
| Score for adherence to mediterranean dietb | 8.6±2.0 | 8.5±2.0 | 8.8±1.9 | 8.7±2.0 |
| Alcohol consumption, g/d | 8.4±13 | 8.1±15 | 9.9±15 | 8.9±13 |
| Waist circumference, cm | ||||
| Women | 98.1±10.2 | 101.89±10. | 98.0±10.3 | 100.7±10.2 |
| Men | 101.2±8.8 | 106.4±9.4 | 102.9±8.9 | 106.0±8.8 |
| Waist circumference> 88cm (women) or> 102cm (men), % | 64.1 | 80.7 | 68.6 | 76.8 |
| Body mass index, kg/m2 | 29.4±3.6 | 31.1±3.8 | 29.8±3.8 | 30.7±3.8 |
| Family history of premature CHD, % | 19.3 | 19.3 | 20.1 | 19.1 |
| Hypertension, % | 82.2 | 87.4 | 82.8 | 88.4 |
| Dyslipidemia, % | 69.0 | 64.1 | 68.4 | 65.2 |
| Type 2 diabetes, % | 52.1 | 59.5 | 49.9 | 47.9 |
| AF, % | 0 | 4.3 | - | - |
| HF, % | - | - | 0.2 | 0.4 |
AF, atrial fibrillation; CHD, coronary heart disease; EVOO, extra-virgin olive oil; HF, heart failure; MET, metabolic equivalent.
Values are expressed as mean±standard deviation or percentage.
Short, medium- and long-chain ACs were highly correlated (). displays the β coefficients of AC scores according to several cardiovascular risk factors. Glucose was associated with short-chain ACs, and triglycerides with medium-chain ACs.
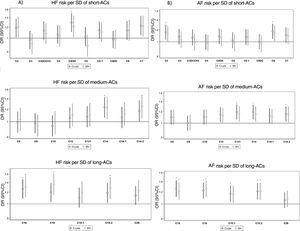
Acylcarnitines and heart failure riskIn the multivariable model, C14, C16, C18, and C18:2 were associated with a higher risk of HF after correction for multiple comparisons (figure 1A and ). Significant results remained the same for C16, C18 and C18:2 when we additionally adjusted for branched-chain amino acids ().
Odds ratios (95% confidence intervals) between baseline AC levels and incident HF or AF in nested case-control studies (cases and controls matched by sex, age, and recruitment center). AC, acylcarnitine; AF, atrial fibrillation; HF, heart failure; MV, multivariable model adjusted for intervention group, body mass index, smoking, leisure-time physical activity, prevalent chronic diseases, and medication use; SD, standard deviation.
During the follow-up, 50 HF cases developed coronary heart disease or cerebrovascular disease before the diagnosis of HF and 54 controls also developed at least 1 of these major CV disease events. No changes were observed in the association between individual ACs and HF when we additionally adjusted for previous incident coronary heart disease or cerebrovascular disease (data not shown).
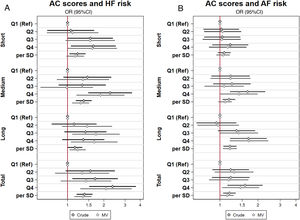
The total score with all ACs was associated with a higher risk of HF (). Higher HF risk was observed for medium- and long-chain AC scores in the comparison between extreme quartiles (figure 2A). Both medium- and long-chain AC scores were significantly associated with HF as continuous variables (). No significant differences for interaction tests were observed when we analyzed the potential effect modification by sex or age group (≤ 70 vs> 70 years) in the association between AC scores and HF risk ().
Association between baseline combined scores (weighted sum of normalized values for each metabolite [using the leave one method to avoid overfitting]) of plasma acylcarnitines and incident HF or AF in nested case-control studies (cases and controls matched by sex, age, and recruitment center) of the PREDIMED trial. AC, acylcarnitine; AF, atrial fibrillation; HF, heart failure; MV, multivariable model adjusted for intervention group, body mass index, smoking, leisure-time physical activity, prevalent chronic diseases, and medication use; Ref, reference; SD, standard deviation.
Regarding AF risk, C16, C18, and C18:2 ACs were associated with a higher AF risk after correction for multiple comparisons (figure 1B and ). These associations did not change when we additionally adjusted for branched-chain amino acids (). A total of 62 AF cases and 73 controls developed coronary heart disease or cerebrovascular disease before diagnosis of AF. No changes were observed when we additionally adjusted for these previous incident cardiovascular diseases (data not shown).
We observed that only LCACs were significantly associated with AF risk, with an OR (95%CI, P for trend) of 1.20 for each SD (1.06-1.36, P for trend=.005) (). No significant effect modification by sex or age group (≤ 70 vs> 70 years) was found ().
Acylcarnitines and composite outcome of heart failure/atrial fibrillationIn the composite outcome of HF or AF cases, a significant association was observed for both medium-chain AC and LCAC scores (). When we mutually adjusted for all AC scores, incident HF was significantly associated only with medium-chain ACs and incident AF only with LCACs ().
In the multinomial logistic regression models (table 2), the odds of developing only HF in comparison with participants without incident HF or AF, was 27% (95%CI, 8%-49%) higher per SD of the medium-chain AC score, which was not significant for those participants who developed only AF and was 40% (95%CI, 9%-78%) higher for those who developed HF and AF per SD of the medium-chain AC score. A similar higher risk was observed for only HF or AF for each SD increase of LCAC score and a significant association was found between the short-chain AC score and only HF risk in comparison with participants without HF or AF.
Odds ratios and 95% confidence intervals from multinomial logistic regression models showing the association between baseline scores of plasma acylcarnitines levels and a composite outcome
| Multivariablea adjusted OR (95%CI) | ||
|---|---|---|
| per 1 SD incrementb | P | |
| No HF or AF (n=726) | ||
| Short-chain AC | 1.00 (ref) | - |
| Medium-chain AC | 1.00 (ref) | - |
| Long-chain AC | 1.00 (ref) | - |
| Total AC | 1.00 (ref) | - |
| Only HF (n=218) | ||
| Short-chain AC | 1.23 (1.02-1.48) | .035 |
| Medium-chain AC | 1.27 (1.08-1.49) | .004 |
| Long-chain AC | 1.22 (1.09-1.38) | .001 |
| Total AC | 1.38 (1.14-1.66) | .001 |
| AF only (n=401) | ||
| Short-chain AC | 1.08 (0.97-1.19) | .158 |
| Medium-chain AC | 1.07 (0.97-1.18) | .154 |
| Long-chain AC | 1.25 (1.09-1.43) | .001 |
| Total AC | 1.17 (1.05-1.30) | .004 |
| AF and HF (n=108) | ||
| Short-chain AC | 0.99 (0.81-1.21) | .909 |
| Medium-chain AC | 1.40 (1.09-1.78) | .007 |
| Long-chain AC | 1.29 (0.98-1.69) | .072 |
| Total AC | 1.31 (0.94-1.84) | .111 |
95%CI, 95% confidence interval; AC, acylcarnitine; AF, atrial fibrillation; EVOO, extra virgin olive oil; HF, heart failure; MedDiet, mediterranean diet; OR, odds ratio; ref, reference; SD, standard deviation.
Adjusted for age, sex, recruitment center, intervention group (MedDiet+EVOO. MedDiet+nuts), body mass index (kg/m2), smoking (never, current, former), leisure-time physical activity (metabolic equivalent tasks in minutes/d), prevalent chronic diseases (dyslipidemia, hypertension, and diabetes), and medication use (angiotensin-converting enzyme inhibitors, diuretics, other antihypertensive treatments, statins and other lipid-lowering agents, insulin, oral hypoglycemic agents and antiplatelet therapy).
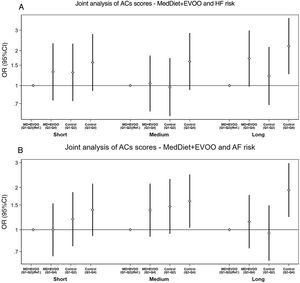
Figure 3 shows the joint analysis for quartiles of AC scores (Q1-Q2 vs Q3-Q4) and the dietary intervention (MedDiet+EVOO vs control group) and the association with HF or AF risk. The MedDiet+EVOO group modified the effect of high levels of LCACs (Q3-Q4) on AF risk compared with the control group (P for additive interaction=.036). No other additive or multiplicative interactions were found according to the intervention groups.
Odds ratioa (95%CI) of the joint analysis of AC scoresb/MedDiet+EVOO and HF or AF risk in nested case-control studies of the PREDIMED trial. A: RERI (95%CI; P value): short-AC,−0.05 (−1.15 to 1.04; P=.924), medium-AC, 0.53 (−0.29 to 1.35, P=.209), long-AC, 0.23 (−0.41 to 0.87; P=.478). B: RERI (95%CI; P value): short-AC, 0.17 (−0.37 to 0.71; P=.534), medium-AC,−0.21 (−0.94 to 0.51, P=.562), long-AC, 0.77 (0.05 to 1.48; P=.036). The MedDiet+nuts group was not included in this analysis. 95%CI, 95% confidence interval; AC, acylcarnitine; AF, atrial fibrillation; EVOO, extra-virgin olive oil; HF, heart failure; MedDiet, mediterranean diet; RERI, relative excess of risk due to interaction. a Odds ratio (95% confidence interval) adjusted for intervention group, body mass index, smoking, leisure-time physical activity, prevalent chronic diseases, and medication use. b Weighted sum of normalized values for each metabolite.
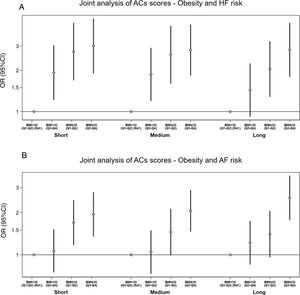
We observed greater AF risk associated with high levels of LCACs (Q3-Q4) among obese participants compared to high levels among nonobese participants (P for additive interaction=.022) (figure 4). No significant interactions were observed except for the association between short-chain ACs and HF in the analysis stratified by T2D.
Odds ratioa (95%CI) of the joint analysis of AC scoresb/obesity and HF or AF risk in nested case-control studies of the PREDIMED trial. A: RERI (95%CI; P value): short-AC,−0.65 (−2.15 to 0.84; P=.392), medium-AC,−0.65 (−2.08 to 0.79, P=.377), long-AC, 0.34 (−0.83 to 1.51; P=.568). B: RERI (95%CI; P value): short-AC, 0.18 (−0.55 to 0.91; P=.634), medium-AC, 0.56 (−0.13 to 1.25, P=.112), long-AC, 0.92 (0.14 to 1.70; P=.022). 95%CI, 95% confidence interval; AC, acylcarnitine; AF, atrial fibrillation; BMI, body mass index; HF, heart failure; RERI, relative excess of risk due to interaction. a Odds ratio (95% confidence interval) adjusted for intervention group, body mass index, smoking, leisure-time physical activity, prevalent chronic diseases, and medication use. b Weighted sum of normalized values for each metabolite.
In 2 matched case-control studies nested within the PREDIMED trial, the risk of incident HF and AF was higher among individuals with elevated levels of LCACs at baseline whereas some short- or medium-chain ACs were more clearly positively associated with HF. Our results also suggest a potential modification of the effect of high levels of LCACs on AF risk by obesity defined as BMI ≥ 30 kg/m2 (increasing the risk), and by a MedDiet intervention supplemented with EVOO (reducing the risk).
Our findings are consistent with some results from previous HF case-control studies. One study identified higher levels of linoleylcarnitine and hydroxybutyrylcarnitine in stage C HF patients compared with controls.12 In the CATHGENE study, higher concentrations of 6 LCACs were found in patients with HF with reduced ejection fraction (HFrEF) than HF with preserved fraction (HFpEF) and in both types of HF, concentrations were higher than in controls.13 In a smaller case-control study, LCAC concentrations were higher in HF patients than in controls.14 Finally, the results of the Mälmo study have shown an association between medium- and long-chain ACs.15 Our study found a stronger association between medium-chain ACs, instead of LCACs, and HF. One reason to explain this discrepancy is that we analyzed the association between baseline ACs and incident HF instead of measuring ACs levels in patients already diagnosed with HF.
In the MURDOCK cohort study, medium-chain ACs, short-chain dicarboxyl ACs and LCACs were associated with incident AF in patients referred for coronary angiography.16 We observed the strongest association between LCACs and AF, whereas the association with short- or medium-chain ACs lost significance after adjustment for different confounders. No detailed information was provided in the MURDOCK study about the specific ACs associated with AF but the authors classified palmitoyl-L-carnitine as a medium-chain AC rather than as an LCAC, as we did in our study. Another difference is that the MURDOCK study included patients who developed AF after coronary artery bypass grafting surgery,16 whereas the PREDIMED trial excluded postoperative incident AF cases.17 The role of LCACs is supported by its known effect of increasing intracellular calcium and inducing electrophysiological alterations in experimental systems where calcium efflux was increased, in a concentration-dependent manner, by palmitoyl-L-carnitine and stearoyl-L-carnitine but not by short-chain esters.18
The analysis and interpretation of plasma ACs levels is complex because increased concentrations have been associated with several chronic diseases. Our participants were at high CV risk, with an average age of 70 years, the mean BMI was 30kg/m2 and> 50% had T2D. All these factors are related to increased plasma AC levels. No significant differences were observed in the association between ACs and HF or AF risk when we stratified by sex or age group (≤ 70 vs> 70 years). These results are in line with a study which found similar total average levels of ACs among men and women.19 However, another study observed sex-specific effects of smoking and BMI on AC metabolism,20 and further research is warranted to explore the potential effects of (or effect modification by) BMI and smoking. We observed associations between AC levels and some cardiovascular risk factors. In a previous study within the PREDIMED trial, we observed that short-chain and LCAC increased the risk of T2D,21 whereas short- and medium-chain plasma ACs were associated with CV disease5 (myocardial infarction, stroke, and CV death). Other studies have shown that increased levels of hydroxybutyrylcarnitine, medium-chain ACs, and oleoylcarnitine were associated with prediabetic status, T2D, and cardiometabolic risk factors.22 A small study found significant positive correlations between ACs and overweight/obesity measures (BMI and waist circumference), and diastolic blood pressure and negative correlation with HDL-cholesterol.23 Further studies with larger sample sizes are needed to explore these associations and to identify potential biomarkers associated with HF and AF risk and the potential modulatory effect of cardiovascular risk factors such as high glycemia and obesity.24,25
Propionylcarnitine and isovalerylcarnitine are byproducts of branched-chain amino acid metabolism, and higher levels of plasma branched-chain amino acids and C3/C5 ACs are found in people with overweight and metabolic syndrome.26 We observed an association between fasting glycemia and short-chain ACs and a stronger association between short- and medium-chain ACs and HF or AF in obese participants, although the interaction was not significant. Moreover, we have previously reported the association between branched-chain amino acids with CV disease27 and T2D.28
Medium- and LCACs are byproducts of mitochondrial energy production through oxidative catabolism of fatty acids. LCACs are mainly involved in muscle and cardiac metabolism and it has been suggested that heart may be the major contributor for LCACs in plasma.29 The accumulation of LCACs in the diabetic myocardium reflects alterations in the fatty acids as a substrate for energy.30 During the development of HF, the ability of the heart to use fatty acids as the main energy source is impaired and ketone bodies are used as alternative fuel sources.31 This mitochondrial dysfunction has been proposed as a common molecular mechanism between both HF and AF based on animal models.32
The associations between LCAC and HF or AF were stronger among obese participants and among those assigned to the control group. These associations may be explained by the systemic chronic inflammation related to obesity and the anti-inflammatory effect of the MedDiet and EVOO. The accumulation of LCACs is involved in the activation of proinflammatory signaling pathways and systemic inflammation is a common mechanistic process for HF and AF.33 This inflammatory process is linked to the expansion of the epicardial adipose tissue which is involved in the pathogenesis of AF34 and it is prominent in patients with HFpEF and AF.3 However, more research is needed to better understand if the potential protective effect of the MedDiet+EVOO occurs through a modulation of the effect of fatty acid oxidation on AF or through a mitigation of the inflammatory effect of LCACs. Moreover, a trial conducted in Spain found an association between a MedDiet intervention and changes in circulating metabolites, including ACs, and these changes were associated with decreases in glucose, insulin, and HOMA-IR.35 In addition, this study suggested an interplay between diet, circulating metabolites and gut microbiota. A major strength in our study is the prospective design of 2 matched case-control studies nested within the PREDIMED trial, which allowed the preservation of the adequate temporal sequence because we measured plasma ACs years earlier than the development of incident HF and AF. Another strength is the ability to control for a wide number of potential confounders. In addition, because of the randomized design, we were able to explore whether the observed associations were modified by the MedDiet interventions.
LimitationsOur study has also several limitations. First, most participants who developed HF could not be defined as HFrEF or HFpEF patients. Other studies have observed differences in the association between ACs levels and HFrEF or HFpEF.13 Thus, future research is needed to explore whether the overlap between HF and AF depends on the type of HF as well as the association with the MedDiet enriched with EVOO. Second, the number of cases was relatively low, especially for the number of HF cases and the overlap between cases of HF and AF. Similarly, the study of potential interactions mediated by obesity or diet would need studies with a larger sample size. However, our study has a unique design to explore the effect modification of the MedDiet and reporting of both additive and multiplicative estimates was encouraged. Third, we identified a limited number of ACs and further studies are needed to examine whether other ACs are associated with HF and AF. However, our results have confirmed previous associations between some individual ACs that we measured and the risk of AF and HF. Fourth, we did not quantify absolute concentrations of ACs and the practical clinical implications will probably need further assessments in the clinical setting. However, our results provide new insights into the common and differential associations between ACs and HF or AF and the potential mechanisms associated with the MedDiet. Finally, we cannot extrapolate our results to other populations with different age or prevalence of cardiovascular risk factors.
CONCLUSIONSAmong a population of individuals at high risk of cardiovascular disease, baseline LCACs were significantly associated with an increased risk of incident HF and AF, and some short- or medium-chain ACs exhibited stronger positive associations with HF. The highest risk in association with increased ACs was observed for developing both AF and HF. An intervention with the MedDiet+EVOO might attenuate the detrimental effect of LCACs on AF risk.
- -
Fatty acid metabolic dysregulation in mitochondria is a common mechanism involved in the development of heart failure (HF) and atrial fibrillation (AF), and elevated acylcarnitines are associated with a higher risk of both diseases.
- -
Elevated plasma long-chain ACs (LCACs) in high cardiovascular risk patients is associated with a higher risk of incident HF and AF. An additive interaction of the association between LCACs and AF risk by the MedDiet supplemented with extra-virgin olive oil and by obesity was observed in an inverse and direct manner, respectively.
This work was supported by National Institutes of Health research grant R01HL118264. The PREvención con DIeta MEDiterránea trial was supported by the official funding agency for biomedical research of the Spanish government, Instituto de Salud Carlos III (ISCIII), through grants provided to research networks specifically developed for the trial (RTIC G03/140, to Ramón Estruch from 2003-2005; RTIC RD 06/0045, to Miguel A. Martínez-González from 2006-2013 and through Centro de Investigación Biomédica en Red de Fisiopatología de la Obesidad y Nutrición [CIBEROBN]), and by grants from Centro Nacional de Investigaciones Cardiovasculares (CNIC 06/2007), Fondo de Investigación Sanitaria–Fondo Europeo de Desarrollo Regional (PI04–2239, PI 05/2584, CP06/00100, PI07/0240, PI07/1138, PI07/0954, PI 07/0473, PI10/01407, PI10/02658, PI11/01647, P11/02505, PI13/00462, and JR17/00022), Ministerio de Ciencia e Innovación (AGL-2009–13906-C02, AGL2010–22319-C03 and SAF2016–80532-R), Fundación Mapfre 2010, Consejería de Salud de la Junta de Andalucía (PI0105/2007), Public Health Division of the Department of Health of the Autonomous Government of Catalonia, Generalitat Valenciana (ACOMP06109, GVA-COMP2010–181, GVACOMP2011–151, CS2010-AP-111, PROMETEO 17/2017 and CS2011-AP-042), Fundació La Marató-TV3 (grants 294/C/2015 and 538/U/2016) and Regional Government of Navarre (P27/2011). Dr Marta Guasch-Ferré was supported by American Diabetes Association grant #1-18-PMF-029. Prof. Jordi Salas-Salvadó is partially supported by ICREA under the ICREA Academia program.
AUTHORS’ CONTRIBUTIONSM. Ruiz-Canela conducted the statistical analyses, drafted the article and is responsible for the overall content as guarantor. M. Guasch-Ferré, F.B. Hu, E. Toledo, C. Razquin, P. Hernández, C.B. Clish, L. Liang, C. Wittenbecher, J. Salas-Salvadó, and M.Á. Martínez-González made substantial contributions to the conception and design of the work. All authors contributed substantially in the acquisition of data, and analysis or interpretation of the data. All authors revised the article critically for important intellectual content and approved the final version.
CONFLICTS OF INTERESTDr Salas-Salvadó declares that he is an unpaid member of the Nut and Dried Fruit Foundation and Danone Institute International, he is a member of the Danone Institute, Spain, and he is partially supported by ICREA under the ICREA Academia programme. The remaining authors declare they have no conflicts of interest. Role of the sponsors: None of the funding sources played a role in the design, collection, analysis, or interpretation of the data or in the decision to submit the manuscript for publication.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2021.10.005


![Association between baseline combined scores (weighted sum of normalized values for each metabolite [using the leave one method to avoid overfitting]) of plasma acylcarnitines and incident HF or AF in nested case-control studies (cases and controls matched by sex, age, and recruitment center) of the PREDIMED trial. AC, acylcarnitine; AF, atrial fibrillation; HF, heart failure; MV, multivariable model adjusted for intervention group, body mass index, smoking, leisure-time physical activity, prevalent chronic diseases, and medication use; Ref, reference; SD, standard deviation. Association between baseline combined scores (weighted sum of normalized values for each metabolite [using the leave one method to avoid overfitting]) of plasma acylcarnitines and incident HF or AF in nested case-control studies (cases and controls matched by sex, age, and recruitment center) of the PREDIMED trial. AC, acylcarnitine; AF, atrial fibrillation; HF, heart failure; MV, multivariable model adjusted for intervention group, body mass index, smoking, leisure-time physical activity, prevalent chronic diseases, and medication use; Ref, reference; SD, standard deviation.](https://static.elsevier.es/multimedia/18855857/0000007500000008/v1_202207230616/S1885585721003133/v1_202207230616/en/main.assets/thumbnail/gr2.jpeg?xkr=eyJpdiI6IkhZenlIbUtaQjk4MHpuMFlFUEEzQmc9PSIsInZhbHVlIjoiNVVnYXhHNVlDSkE0MU5vT243bjNNaERpQTl2ck9hOENpREt2R2g0cVM4az0iLCJtYWMiOiI0OTgzNmQwMGNkNDg0YmUxYzcwNjBkNjhmOGUwODBiYzMzOWViOGYxYThmZDFiZmQ4NWVhYmFkYjFkMTFhNDZiIiwidGFnIjoiIn0=)

