Revista Española de Cardiología recently published the results of 3 retrospective studies of patients with atrial fibrillation in Spain. The 3 studies evaluate the quality of anticoagulation control over 1 year of primary care therapy with vitamin-K antagonists. In all studies, the majority of patients received acenocoumarol therapy and 40% of patients had inadequate anticoagulation control.
In the ANFAGAL study (ANticoagulación en pacientes con Fibrilación Auricular en el ámbito de atención primaria de GALicia) study, conducted in Galicia, Cinza-Sanjurjo et al1 studied 511 primary care patients with nonvalvular atrial fibrillation who received anticoagulant therapy with vitamin-K antagonists for more than 1 year. Overall, 41.5% of patients were in the therapeutic range on fewer than 60% of follow-up visits. This group of patients took more medication and had higher incidences of kidney disease and major bleeding. Furthermore, the study excluded patients who required temporary interruption of anticoagulation therapy, as this increases the proportion of patients with poorly controlled anticoagulation.
The other 2 studies are larger and national in scope. The CALIFA2 study included 1542 patients who were followed-up by cardiologists and who were receiving treatment for chronic atrial fibrillation, and adequate anticoagulation control was achieved in 60% of cases. The last study, PAULA,3 evaluated 1524 patients, and anticoagulation was in the therapeutic range during 60% of the follow-up period.
POOR ANTICOAGULATION CONTROL WITH VITAMIN-K ANTAGONISTSPoor anticoagulation control was related to diverse factors that did not fully overlap between the 3 registries. Associated factors included kidney failure, female sex, a history of poor control, dietary habits, concomitant therapy with antiplatelet drugs, and long-term use of nonsteroidal antiinflammatory drugs; none of these factors is easily amended or accommodated in standard clinical practice.
The results of the 3 studies are remarkably similar and are in agreement with other contemporary observations in registries and also in clinical trials, in which participants in principle have higher treatment adherence and the controls are stricter. The most important observation is the persistent inability to maintain anticoagulation within the therapeutic range, even today, when patient training and the organization of follow-up checks are better than they were formerly. Anticoagulation control with vitamin-K antagonists is highly unlikely to improve significantly in the near future. More important still, time in therapeutic range is not routinely measured in all patients, and therefore this parameter is still not included as standard in clinical histories.
THE IMPORTANCE OF ADEQUATE ANTICOAGULATION CONTROLThe therapeutic range for anticoagulation with vitamin-K antagonists is very narrow,4 and an international normalized ratio (INR) outside the range of 2.0 to 3.0 recommended for atrial fibrillation markedly increases the risk of embolic events, major bleeding episodes, and even death.5 Numerous studies have shown that the time in therapeutic range needs to exceed 70% in order to significantly reduce the risk of stroke in patients with a CHADS2 score >2, although a poorer level of anticoagulation is always related to a higher risk.5 All physicians with responsibility for managing patients’ anticoagulation will be aware of this relationship. However, we tend not to follow through with the implementation of all the measures at our disposal; instead we limit ourselves to repeating INR tests and adjusting anticoagulant doses in an attempt to improve outcomes. While this is sometimes achieved in isolated cases, there has been no overall improvement in recent years. A very common, though not inevitable, consequence of poor control is discontinuation of anticoagulation medication, especially when there is major bleeding.6 Worse still, poorly controlled anticoagulation is generally very well tolerated, especially when the INR is < 2 (absence of effective anticoagulation) and the patient remains asymptomatic. It would be interesting to know what therapeutic attitude was adopted toward the patients with poor anticoagulation in the 3 studies published in Revista Española de Cardiología.
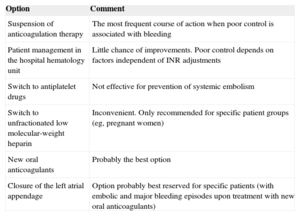
POSSIBLE SOLUTIONSTable 1 lists possible approaches to reducing the risk of embolism and bleeding.
Treatment Options for Atrial Fibrillation Patients With Poorly Controlled Anticoagulation
| Option | Comment |
|---|---|
| Suspension of anticoagulation therapy | The most frequent course of action when poor control is associated with bleeding |
| Patient management in the hospital hematology unit | Little chance of improvements. Poor control depends on factors independent of INR adjustments |
| Switch to antiplatelet drugs | Not effective for prevention of systemic embolism |
| Switch to unfractionated low molecular-weight heparin | Inconvenient. Only recommended for specific patient groups (eg, pregnant women) |
| New oral anticoagulants | Probably the best option |
| Closure of the left atrial appendage | Option probably best reserved for specific patients (with embolic and major bleeding episodes upon treatment with new oral anticoagulants) |
INR, international normalized ratio.
Suspension of anticoagulation therapy is justified only in cases of major bleeding, and then only as a temporary measure before the implementation of an alternative strategy. Antiplatelet drugs are inappropriate for the prevention of embolic risk in atrial fibrillation. Switching from vitamin-K antagonists to another anticoagulant, usually unfractionated heparin or enoxaparin, is a strategy adopted in special cases of difficult anticoagulation in patients at high risk (eg, pregnant women or patients with a mechanical prosthesis). Adjustment to achieve a dose within the therapeutic range is more effective with these drugs; however, the procedure is more complex and generally requires meticulous management by an anticoagulation specialist.
At the opposite end of the treatment spectrum is percutaneous closure of the left atrial appendage, the site of thrombus formation in more than 90% of cases of atrial fibrillation. This method is complex, experience is limited, and the occlusion devices are still evolving; nonetheless, left atrial appendage closure is clearly an effective procedure that should be considered for patients with poor anticoagulation control and repeated embolic and major bleeding episodes, especially if these occur with the most effective anticoagulation drugs available: the new generation of oral anticoagulants.
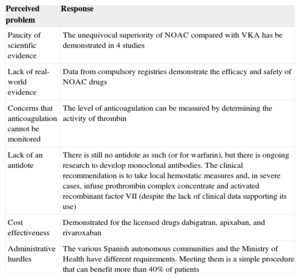
CONCLUSIONSWithout doubt, the best therapeutic option today is the new generation of oral anticoagulants, such as the thrombin inhibitor dabigatran and the direct factor Xa inhibitors apixaban, rivaroxaban, and, when its clinical use is indicated, edoxaban. Studies with these drugs are not directly comparable; however, all four drugs have demonstrated clinical efficacy compared with warfarin,7–10 anticoagulation is uniform and reliable from the first dose (thus avoiding the need for routine INR testing), and guidelines recommend their use as a first-line anticoagulation therapy if they are available.11 Despite their advantages, new anticoagulants have a much lower uptake in clinical practice than expected, and in Spain are used to treat only 10% to 15% of patients, depending on the Spanish autonomous community. While there are many reasons for this low uptake of the new oral anticoagulants, none of them is fully justified, and in fact physicians’ concerns about these drugs are for the most part misplaced (Table 2). The efficacy of these drugs is beyond dispute and the results of studies are consistent internally and among all subgroups of the 100000 patients included in contemporary clinical trials; this contrasts sharply with the complete lack of controlled clinical trials with acenocoumarol, the most widely used vitamin-K antagonist in Spain. Despite perceptions to the contrary, the level of anticoagulation with the new drugs can be tested, using calibrated anti-factor Xa for rivaroxaban or apixaban and thrombin coagulation time for dabigatran. However, systematic testing is unnecessary, and tests should only be used when needed, for example when there is bleeding.12 Also unjustified is the fear of losing anticoagulation upon missing 1 dose, especially when compared with the absence of anticoagulation for days or months that occurs with warfarin or acenocoumarol if the INR is below the lower therapeutic limit. When there is bleeding, it is possible to reverse the anticoagulation, by administering prothrombin complex concentrate, clotting factor VII, and mixtures of coagulation factors that require consultation with hematologists to ensure their optimal use. Reversing anticoagulation is in fact easier and faster with the new oral anticoagulants. Moreover, research into specific antidotes is highly advanced, and in some cases has already yielded definitive clinical results. This leaves the question of cost and administrative difficulties. The new anticoagulants that are already in clinical use are cost effective. It is true that financing from the Spanish National Health System for the new anticoagulants is limited to special cases, but these include contraindications (eg, severe kidney disease, heart valve disease), and poor anticoagulation control is one of the uses approved for health service funding.13 If many patients are still poorly controlled with acenocoumarol or warfarin, some of the fault lies with us, the physicians. How much longer?
Perceived Problems Underlying the Low Prescription of New Oral Anticoagulants
| Perceived problem | Response |
|---|---|
| Paucity of scientific evidence | The unequivocal superiority of NOAC compared with VKA has be demonstrated in 4 studies |
| Lack of real-world evidence | Data from compulsory registries demonstrate the efficacy and safety of NOAC drugs |
| Concerns that anticoagulation cannot be monitored | The level of anticoagulation can be measured by determining the activity of thrombin |
| Lack of an antidote | There is still no antidote as such (or for warfarin), but there is ongoing research to develop monoclonal antibodies. The clinical recommendation is to take local hemostatic measures and, in severe cases, infuse prothrombin complex concentrate and activated recombinant factor VII (despite the lack of clinical data supporting its use) |
| Cost effectiveness | Demonstrated for the licensed drugs dabigatran, apixaban, and rivaroxaban |
| Administrative hurdles | The various Spanish autonomous communities and the Ministry of Health have different requirements. Meeting them is a simple procedure that can benefit more than 40% of patients |
VKA, vitamin-K antagonists; NOAC, new oral anticoagulants.
J. López-Sendón has received significant payments for fellowships, consultancy work, or conference presentations from Pfizer, Boheringher, Bayer, and Daychii Sankyo. J.L. Merino has received significant payments as an advisory board member or for consultancy work, conference presentations, or endorsement of educational activities or equipment from Daichii-Sankyo, Bayer, Boheringher, Pfizer, Cardiome, Sanofi, Biosense, Magnetecs, Biotronik, Boston Scientific, Medtronic, St. Jude Medical, Sorin, and Microport.