Keywords
INTRODUCTION
In patients who have suffered ST-segment elevation acute myocardial infarction (STEMI), the existence of microvascular obstruction (MVO) after different reperfusion therapies is associated with a poorer prognosis.1 Leukocytes,2 neutrophils,3 and monocytes4 have been defined as the mediators of this proinflammatory process, against which the different therapeutic strategies so far tried in humans have had very little success.5 If new therapeutic strategies are to be designed, better knowledge of the physiopathology of MVO will be necessary.
Recent studies indicate that in serious clinical conditions such as septic shock or extensive burns, poor progress is less related to the proinflammatory reaction than to severe and persistent immunodeficiency. The latter can be easily identified by the existence of lymphopenia.6 The lymphocytes have an important role in the control of the inflammatory system and the progression of atherosclerosis.7,8 This might explain the increase in the incidence of cardiovascular diseases in immunodeficiency-associated lymphopenia.9 While the existence of leukocytosis, neutrophilia, and monocytosis following STEMI, and the association of these conditions with a poorer prognosis, is well known,2-4 it was not until very recently that lymphocytes were shown to exert an opposite influence to that of other subgroups of luekocytes.10,11 It is therefore now understood that lymphopenia is a predictor of further events in patients who have suffered an infarction. The physiopathological basis of this association has not been defined and its study might allow advances in the search for new therapies.
Currently, cardiac magnetic resonance (CMR) is the reference technique for the complete, imaging-based, non-invasive analysis of the consequences of STEMI.12 This technique provides a reliable estimate of the presence and extent of MVO.13,14 In the present work, CMR was used to confirm the presence of MVO, to study infarction size, and to determine left ventricular volumes and systolic function. The aim of this work was to analyze the relationship between post-reperfusion lymphopenia and the existence of MVO confirmed by CMR in the first week following STEMI.
METHODS
Study Group
The subjects of this prospective study were 260 consecutive patients who, between January 2004 and December 2007, had suffered their first STEMI and received thrombolytic agents or primary angioplasty at our center.
The exclusion criteria were as follows: death (n=14), reinfarction (n=5), or serious clinical instability (n=11) that impeded undergoing CMR in the first week after STEMI. Also excluded were those patients with any contraindication for CMR (n=10), neoplasia, or any acute inflammatory or infectious disease (n=8). The final group therefore consisted of 212 patients who suffered no serious complications during their hospital stay, and who were discharged after CMR was performed.
Reperfusion Therapy
The choice of reperfusion strategy and medical treatment was made by the attending cardiologist. Fifty-eight percent (n=124) of patients received only thrombolytic treatment while 42% (n=88) underwent primary angioplasty. All subjects underwent coronary angiography. A total of 189 (89%) patients received a stent, 88 during primary angioplasty, 23 during rescue angioplasty, and 78 during elective cardiac catheterization performed after thrombolysis (performed at a mean 1 day post-thrombolysis). Thrombolysis in myocardial infarction (TIMI) and myocardial blush values were determined by an expert observer (blinded to the CMR results) using Integris HM3000 software (Philips, Best, The Netherlands). A TIMI score of 3 and a blush score of 2-3 at the end of catheterization were considered normal.15
Baseline Characteristics, Electrocardiogram, and Leukocyte Subtypes
Baseline characteristics and clinical data were recorded for all patients. A 12-lead electrocardiogram was performed before starting reperfusion therapy and then again 2 h later. The percentage resolution of the ST segment elevation was determined.
Troponin I was determined using an immunometric system (Dimension, Dade Behring Inc., Newark, USA) up on the arrival of each patient and again 2, 12, 24, 48, and 96 h after reperfusion therapy.
Leukocytes, neutrophils, lymphocytes, and monocytes were determined sequentially upon each patient's arrival and again at 2, 12, 24, 48, and 96 h after reperfusion therapy.
Cardiac Magnetic Resonance
CMR was performed using a Sonata Magnetom 1.5 T machine (Siemens, Erlangen, Germany). Imaging before discharge was performed at 7 (1) days (at least 48 h after cardiac catheterization) in accordance with our center's protocol.12 All images were acquired using a surface coil during respiratory apnea and synchronized with the electrocardiogram. Two, 3, and 4 camera short axis (every 1 cm) cine images were acquired using steady state free precession sequences (repetition time/echo time 3.2/1.6 ms; angle of inclination 61°; matrix 256´128; slice thickness 6 mm; temporal resolution 26 ms).
After the administration of gadolinium-diethylene-triamino-pentaacetic acid (0.1 mmol/kg) (Magnograf, Juste SAQF, Spain) at a flow velocity of 3 mL/s, late gadolinium images were captured (at least 10 min after adding the contrast medium) following a steady-state free precession sequence (repetition time/echo time 2.5/1.1 ms; angle of inclination 50°; matrix 195´192; slice thickness, 6 mm), with annulment of the myocardial signal.
Six months (178 [12] days) after the infarction, CMR was performed again in 190 patients following the same protocol. This second test could not be performed in 22 patients because of death (n=3), non-fatal myocardial infraction (n=4), implantation of an automatic defibrillator (n=4) or pacemaker (n=2), or patient disinclination (n=9).
Analysis of Cardiac Magnetic Resonance Data
All CMR data were analyzed by an independent observer, blinded to the characteristics of the patients, using QMASS MR 6.1.5 software (Medis, Leiden, The Netherlands). The 17 segments model was employed.16
End-systolic and end-diastolic volumes, along with the ejection fraction, were determined by manually tracing the endocardial outline of the short axis cine images.
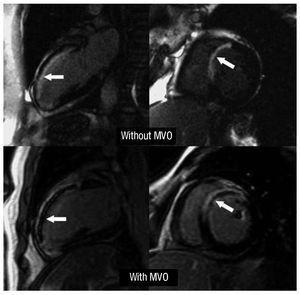
Delayed enhancement was deemed to exist (an indicator of necrosis) if the intensity of the images was >2 standard deviations (SD) over the value of a remote, non-infarcted area in the same slice. The size of the infarction was defined as the mass of the left ventricle that showed delayed enhancement. The existence of MVO in the late contrast sequences was defined as a defect in contrast capture in the center of a segment surrounded by tissue showing delayed enhancement13 (Figure 1). The number of segments (extension) showing MVO was also determined.
Figure 1. Upper panels: example of a patient with an extensive anterior infarction but with no microvascular obstruction (arrows). Lower panels: example of a patient with an extensive anterior infarction with microvascular obstruction (arrows). MVO indicates microvascular obstruction.
Statistical Analysis
The Kolmogorov-Smirnov test was used to determine whether the continuous variables showed a normal distribution. Continuous variables were expressed as the mean (SD) if they met the conditions of normality, or as the median [interquartile range] if the distribution was non-parametric. The Student t test for non-paired data was used to compare the means of continuous variables with a normal distribution; the Wilcoxon or Mann-Whitney U test was used when the variables did not show a normal distribution. Discrete variables were expressed as percentages; comparisons were made using the c2 test.
The independent effect of the post-reperfusion lymphocyte number, using a cut-off defined by the receiver operating characteristic (ROC) curve to predict the existence of MVO, was studied by logistic regression. Multivariate analysis included all variables associated with the presence of MVO that showed a P value of <.2 in univariate analysis. Odds ratios (OR) and 95% confidence intervals (95% CI) were calculated for the variables that were included in the final regression model.
Significance was set at P<.05. All calculations were made using SPSS 11.0 software (SPSS Inc, Chicago, Illinois, USA).
RESULTS
Table 1 shows the baseline characteristics of the 212 patients included in the study.
The existence of MVO was associated with a greater reduction in the ejection fraction (42% [12%] compared to 55% [12%]), a larger end-diastolic volume (92 [31] compared to 74 [20] mL/m2) and end-systolic volume (55 [28] compared to 33 [15] mL/m2), and a larger infarction (38 [16] compared to 20 [10] g) (P<.001 for all comparisons).
A significant reduction was seen in the percentage of patients with MVO between the first week and the sixth month: 67 (32%) of 212 compared to 13 (7%) of 190 patients (P<.001).
Post-Reperfusion Lymphopenia and Microvascular Obstruction
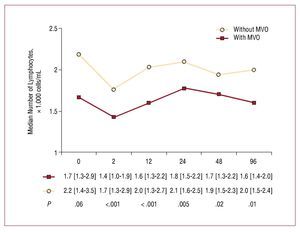
A severe reduction was seen in the number of lymphocytes after reperfusion treatment (2070 [1337-3285] cells/mL before reperfusion compared to 1635 [1160-2347] cells/mL after reperfusion; P<.001). Patients with MVO showed a smaller number of post-reperfusion lymphocytes (1430 [950-1880] cells/mL compared to 1660 [1260-2910] cells/mL in patients with no MVO; P<.001), a difference that persisted over the next hours and even days (Figure 2).
Figure 2. Change in lymphocyte number over time with respect to the existence (or not) of microvascular obstruction. MVO indicates microvascular obstruction.
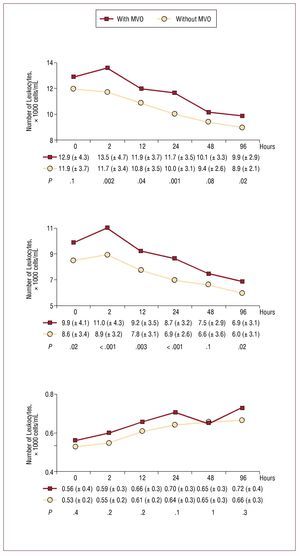
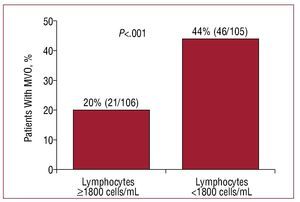
With respect to leukocytes and neutrophils, the opposite was seen (Figure 3), ie, MVO was associated with larger numbers of these cells. The number of monocytes increased progressively following reperfusion, but was not related to MVO (Figure 3). Those patients with a post-reperfusion lymphocyte number of <1800 cells/mL were more likely to show MVO (44% compared to 20% of those with ≥1800 cells/mL; P<.001) (Figure 4).
Figure 3. Change in leukocyte, neutrophil, and monocyte numbers with respect to the existence (or not) of microvascular obstruction (MVO).
Figure 4.Percentage of patients with microvascular obstruction (MVO) with respect to the post-reperfusion lymphocyte count.
Microvascular obstruction was seen in 29% (36/124) of patients who received thrombolysis, and in 35% (31/88) of those who received primary angioplasty (P=.3). A post-reperfusion lymphocyte count of <1800 cells/mL was associated with a greater risk of MVO, both in patients treated with thrombolytic agents (42% compared to 17% in those with >1800 cells/mL); P=.003) and those reperfused via primary angioplasty (47% compared to 23% in those with >1800 cells/mL; P=.02).
A post-reperfusion lymphocyte count of <1800 cells/mL was associated with a more reduced ejection fraction, more dilated left ventricular volumes, larger infarctions, and more extensive MVO both in the first week and sixth month of follow-up (Table 2).
During the first year of follow-up 4 patients died of cardiac causes, 6 suffered a further infarction, and 10 were admitted for heart failure. These patients (n=20) tended to have a lower post-reperfusion lymphocyte count (1385 [882-2032] cells/mL compared to 1670 [1192-2390] cells/mL in those who suffered no event; P=.1).
Multivariate Analysis
Multivariate analysis included all those variables which in univariate analysis returned P values of <.2 (heart rate, Killip class >1, anterior infarction, myocardial blush, percentage resolution of the ST-segment, troponin I level, number of leukocytes, neutrophils, and monocytes). A post-reperfusion lymphocyte number of <1800 cells/mL independently increased the risk of MVO (OR=3.2 [1.6-6.3]; P<.001) (Table 3). A second multivariate study was performed in which, instead of the above variables, all those in Table 1 were included owing to their potential influence on the appearance of MVO, with no appreciable modification to the results shown in Table 3.
DISCUSSION
The most important observation made in this study is that, in STEMI, post-reperfusion lymphopenia is independently associated with the existence of MVO.
In agreement with that reported by other authors,1,14 the failure to restore microvascular flow has negative effects on systolic function and is associated with a larger infarction and a larger left ventricular volume.
Microvascular Obstruction and Leukocyte Subtypes
Most studies performed to date that have analyzed the effects of inflammatory cells on the process of MVO following infarction have focused on "total leukocytes," and have measured these only once at the time of patient admittance.2,17 However, the total number of leukocytes represents all the different subgroups together, and in the acute phase of STEMI very great changes are seen in the numbers of each subgroup,11 changes that are not reflected by a single measurement.
In patients with STEMI, baseline neutrophilia predicts a larger infarction, abnormal coronary perfusion, and a greater risk of death.3,17 The results of the present work show that an increase in the number of neutrophils after reperfusion therapy is associated with a greater risk of MVO.
In a group of 238 patients with infarction, Mariani et al4 observed that monocytosis was associated with a poorer restoration of systolic function at 6 months. In the present study, a progressive increase in the number of monocytes was seen, but no significant relationship with microvascular perfusion was observed. This indicates that the role of monocytes is more related to the repair process once the acute phase is complete. While the relationship between leukocytes in general and neutrophils in particular and an increased risk of MVO is well established, it is important to point out that pharmacological measures directed towards inhibiting the proinflammatory cascade mediated by these cells have failed in human trials.5 This indicates that these cells are more a marker of extensive necrosis than its causal agent, although their rapid increase in number—especially the number of neutrophils—after the onset of spontaneous infarction makes it impossible to effectively prevent their harmful effects in humans. Finally, it may be that neutrophils are not the most appropriate therapeutic objective. The close relationship between lymphopenia and MVO detected in this work could open a door towards a better understanding of the pathophysiology of MVO and the exploration of new therapeutic targets.
Lymphocytes and Microvascular Obstruction
Acute lymphopenia occurs in patients with STEMI,11 but its prognostic and pathophysiological implications have not been well defined. Recently, Dragu et al10 and Núñez et al11 observed that, in patients with acute myocardial infarction, a low baseline number of lymphocytes was associated with a greater risk of death over the following months.
Although systematic and sequential determinations of the hemogram are made following a STEMI, little attention is paid to the course of the lymphocytes, whether to assess the success of reperfusion or stratify risk. Indeed, earlier studies, such as that of Mariani et al,4 focused on the peak numbers of leukocyte subtypes. Since lymphocytes follow a course opposite to the remaining subtypes (towards lymphopenia) this might explain why these authors detected no correlation between the lymphocyte peak and the state of the microcirculation. The present study is the first to report that a severe reduction in the number of lymphocytes following reperfusion is an independent predictor of a failure in microvascular perfusion, even after exhaustive adjustment for other variables. Bearing in mind the ease and the low cost with which this variable can be measured, from a clinical point of view it would be very recommendable that the course taken by lymphocytes be considered a means of assessing the success of reperfusion therapy.
From a practical standpoint, information that allows the success of reperfusion treatments to be evaluated is of more interest just after treatment has begun, when the most significant differences between patients with and without MVO are seen with respect to their ECG, troponin I, and leukocyte subtype data. For this reason, and to simplify the analysis, only the first post-reperfusion measurements of the selected variables (2 h after thrombolysis or primary angioplasty) were included in multivariate analysis.
Apart from its clinical value, the relationship between the immunosuppressive response (detectable in the tendency for lymphopenia to appear) following STEMI and the increased risk of microvascular damage deserves some brief pathophysiological discussion (see below).
Pathophysiological Considerations
In serious conditions, such as sepsis6 or STEMI,7,18 the inflammatory process plays a crucial role, and the ultimate cause of poor clinical progress appears to be linked to an acute loss of regulatory control of the immune system. Following a short neutrophilic response, patients with the poorest prognosis show marked and long-lasting lymphopenia.6,10,11
The physiopathology of the loss of control of the immune system that occurs with STEMI is not well understood. A reduced population of regulatory T cells would appear to be important in this process.7,8,18-21 The main function of these cells is to inhibit an excessive and inappropriate inflammatory response in the face of different stimuli. Following an "inverse pyramid"19 model, the loss of control of the immune system by these cells at the very base of this hypothetical pyramid would lead to an overly large inflammatory response in all the upper echelons of the inflammatory cascade.
One of the mechanisms that regulatory T cells use to inhibit inadequate immune responses is the induction of apoptosis in proinflammatory effector T cells (T helper 1 cells), perhaps as a self-protective response to defend the organism from a storm of inflammatory cytokines.18,20 This might be related to the onset of the severe lymphopenia observed in patients with STEMI. However, the lack of regulation of the immune system might cause not just a loss of T helper 1 cells but also of T helper 2 anti-inflammatory lymphocytes. This would lead to a worsening of the harmful effects of the inflammatory cascade.6-8,18-21 Microvascular and myocardial damage, along with clinical events, could be the final result of this uncontrolled immune response.
Unlike myocardial necrosis, which appears quickly, MVO is a slower process that may take days to become established.1,16 It may therefore be associated with a much wider therapeutic window. This knowledge, plus the relationship found between microvascular damage and severe persistent lymphopenia following reperfusion (a reflection of an acute loss of control over the immune system), may inspire new lines of research that might help us better understand the physiopathology of acute myocardial infarction, and aid in the search for new pharmacological therapies.
Limitations
The number of patients in this study is insufficiently large to allow the clinical implications of lymphopenia in STEMI to be analyzed. An in-depth analysis of all the lymphocyte sub-populations and their products is required if the relationship between the immune response and MVO is to be further understood.
CONCLUSIONS
In patients with STEMI who undergo reperfusion therapy, the existence of severe lymphopenia is associated with a greater risk of MVO. From a clinical point of view, monitoring the change in lymphocyte numbers provides a simple and inexpensive index for determining the success of microvascular reperfusion. From a pathophysiological standpoint, the present results encourage the undertaking of new studies to clarify the role of the immune system in microvascular damage following an infarction, studies that could lead to new treatments.
ABBREVIATIONS
CMR: cardiac magnetic resonance
MVO: microvascular obstruction
STEMI: ST-segment elevation acute myocardial infarction
TIMI: thrombolysis in myocardial infarction
This study was funded by the Sociedad Española de Cardiología (grant FEC) and the Instituto de Salud Carlos III (grants PI 080128 and Heracles).
Correspondence: Dr. V. Bodí.
Servicio de Cardiología. Hospital Clínico y Universitario. Blasco Ibáñez, 17. 46010 Valencia. España.
E-mail: vicentbodi@hotmail.com
Received January 14, 2009.
Accepted for publication June 17, 2009.