Keywords
INTRODUCTION
Despite progress in diagnostic and surgical techniques and postoperative care, acute type-A aortic dissection continues to be associated with high morbidity and mortality in the short- and long-term. Current hospital mortality has been estimated between 15% and 35%, with a 5-year survival rate of 65%-75%.1-7
The high incidence of postoperative stroke in these patients (10%-20%)3,5,7,8 has been associated with inadequate cerebral protection during circulatory arrest, embolic events, or malperfusion due to preferential flow through the false lumen during perfusion using the femoral artery.9,10 In recent years, the introduction of new cerebral protection techniques, such as selective antegrade cerebral perfusion and axillary artery cannulation, has considerably reduced the incidence of this complication.10-17
The aim of this retrospective study is to describe our experience with acute type A aortic dissection surgery— in relation to hospital mortality, the incidence of reintervention and long-term survival—and analyze the influence of cerebral protection on our results.
METHODS
Between March 1990 and October 2007, 98 consecutive patients (79 men and 19 women) underwent surgery in our hospital for acute type-A aortic dissection. Of the patients diagnosed and referred for surgery (103) during this period, 5 were excluded; 2 patients due to being more than 85 years and the others because of presenting irreversible cerebral or visceral lesions.
The median age was 59 years. The most frequent symptom at presentation was acute chest pain. The diagnosis was confirmed through aortography during the initial period (18%), transthoracic echocardiography (76%), transesophageal echocardiography (66%), and thoracoabdominal computerized tomography (CT) (65%). Except for 11 patients, where a single diagnostic test was performed, all the patients were diagnosed using 2 or 3 of these imaging techniques in combination (currently, the diagnostic system employed is thoracoabdominal CT with or without transthoracic echocardiography and intraoperative transesophageal echocardiography).
A total of 91 (93%) patients underwent emergency surgery (within 24 h) and the others underwent urgent surgery (in the 72 h following diagnosis).
The imaging tests demonstrated aortic regurgitation in 83 patients (55% with severe regurgitation). The preoperative clinical and demographic characteristics are shown in Table 1.
Surgical Technique
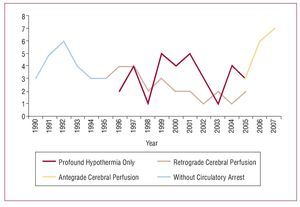
The basic concepts of the surgical procedure involve replacing the ascending aorta or aortic arch, resection of the primary tear and preparation of the distal anastomosis during circulatory arrest. During this 18-year period, there has been progressive evolution in the surgical strategy employed for this pathology. Initially, only the ascending aorta was resectioned with aortic clamping without circulatory arrest. In 1994, distal anastomosis with circulatory arrest was introduced, which is the technique currently used in all patients, while applying different methods of cerebral protection. Selective antegrade cerebral perfusion via the axillary artery was recently introduced as a cerebral protection method during circulatory arrest (Figure 1).
Figure 1. Evolution of the surgical strategy.
This was performed via median sternotomy, femoral artery cannulation (82%), axillary artery cannulation (16%), or aortic arch cannulation (2%), with venous return through the right atrium (85%) or femoral vein (15%) and cardiopulmonary bypass. Body temperature was monitored via the esophagus and bladder, and profound hypothermia was achieved when the bladder temperature reached 18oC.
After aortic clamping, the ascending aorta was opened longitudinally and supracoronary transection performed to locate the intimal tear, if any, and to examine the morphology and functioning of the aortic valve. Then, in profound hypothermia and circulatory arrest, the ascending aorta was unclamped and the entire aortic arch examined. In 27% of patients, retrograde cerebral perfusion was added via the superior vena cava (200-300 mL/min). In the 16 patients where antegrade cerebral protection was performed, the right axillary artery was used (10-15 mL/kg/min), and selective perfusion performed via the left carotid artery in all patients.
The primary tear was identified in 83 patients; it was located in the aortic root in 14%, the ascending aorta in 67%, and the aortic arch in 19% of the patients. The aortic segment affected by the primary tear was resected and replaced with a Hemashield dacron graft (Boston Scientific, Massachusetts, USA) anastomized with continuous 4/0 monofilament suture supported by a heterologous pericardial strip and reinforcing the proximal and distal native aorta with gelatin-resorcin-formaldehyde (GRF, biological glue; Cardial Laboratories, Saint-Etienne, France). During our early experience, aortic segment replacement was performed using the inclusion technique (covering the prosthetic tube with the resected native aorta) in 21 patients. Currently, the graft interposition technique is used (replacement by tubular prosthesis with complete resection of the native aorta).
Replacement of the ascending aorta only was performed in 61 (63%) patients, extended to the hemiarch in 24 (24%), and to the total aortic arch in 13 (13%). By hemiarch, we refer to the cases of partial resection of the arch, with a distal anastomosis only, without the need for reimplantation of the supra-aortic trunk. An elephant trunk was used in 6 patients (during total replacement of the aortic arch, a free segment of the prosthetic tube remains in the distal anastomosis hanging in the descending thoracic aorta, which facilitates potential interventions in this area). The aortic valve was spared by resuspending the commissures with 4/0 monofilament sutures supported by a teflon patch in 46 patients (47%), replaced in 34 (35%) and reimplanted in 1 (1%). When the aortic valve needed replacing, in 73% of patients this was done using a valve graft with reimplantation of the coronary arteries using the Bentall technique. The preoperative condition of the aortic valve is described in Table 2. After surgery, in cases of resuspension, valve competence was objectified through intraoperative transesophageal echocardiography in 84% of patients, mild regurgitation in 13%, and moderate regurgitation (II/IV) in the remaining patients.
Mean cardiopulmonary bypass time was 183 (58) min, ischemia time, 113 (39) min, and circulatory arrest time, 3 (23) min.
A total of 24% of patients, comprising the initial experience, did not undergo circulatory arrest. In the remaining patients, the cerebral protection method used was profound hypothermia in only 32 (33%) patients, retrograde cerebral perfusion was added in 26 (27%) patients, and antegrade perfusion in the other 16 (16%).
Follow-up
All the surviving patients underwent annual clinical and echocardiographic checkups, as well as serial CT examinations to evaluate the distal aorta. Clinical data were obtained by personal and telephone interviews with the patients, family members, and primary care physicians. A 95% follow-up rate was achieved (79 patients).
Statistical Analysis
The SPSS statistical program (version 14.0 for Windows) was used in all the analyses. A univariate analysis was performed on the perioperative variables to determine the statistically significant risk factors (P<.05) for hospital mortality, reintervention and mortality during follow-up. The results underwent logistic regression analysis or Cox regression analysis to determine the independent risk factors. Kaplan-Meier survival curves were constructed to estimate the absence of reinterventions and survival as a function of time.
RESULTS
Hospital Mortality
Hospital mortality was 15% (15/98): 6 patients died due to intraoperative bleeding; 3, low cardiac output; 2, neurological damage; 2, sepsis; and 2 due to postoperative multiorgan failure. Table 3 shows the postoperative complications.
The univariate analysis showed that old age, left ventricular dysfunction, cardiogenic shock, cardiopulmonary bypass time >200 min, aortic clamping time >130 min, and postoperative stroke were risk factors for hospital mortality (Table 4). These variables underwent multivariate analysis which showed that old age (≥70 years) (RR=2.85; P=.04) and preoperative cardiogenic shock (RR=2.6; P=.025) were identified as independent predictors of hospital mortality.
Preoperative neurological dysfunction and the first years of experience (1990-1994) were the only variables that were associated with postoperative neurological complications (Table 4) in the univariate analysis, but these did not reach statistical significance in the multivariate analysis.
Mortality and specific postoperative neurological complications (permanent stroke and temporary neurological dysfunction [TND]) were measured among the patients who underwent circulatory arrest and those who did not and were stratified according to the method of cerebral protection used (profound hypothermia alone or in combination with retrograde or antegrade perfusion) (Table 5). No statistically significant differences were found between any of them.
Follow-up
A 95% follow-up rate (79 patients) was achieved, with a median of 61 (range, 1-204) months. After hospital discharge, all the patients underwent echocardiographic examination at 2 months and annually thereafter. A total of 67% (56/83) of patients underwent at least 1 CT examination. Of the 27 surviving patients who did not undergo CT, 17 refused the test or did not attend, 4 were lost to the study and 6 died during follow-up, 1 due to a known aortic cause (ruptured abdominal aortic aneurysm).
Computerized tomography showed persistent patent false lumen in the distal aorta in 71% of the patients, of whom 17% presented progressive dilatation of the thoracic aorta and abdominal aorta.
Reintervention
In total, 13 (16%) patients underwent reintervention during follow-up. The causes were as follows: severe aortic regurgitation and dilatation of the aortic root in 5 patients, severe aortic regurgitation and redissection of the aortic root in 3, severe aortic regurgitation alone in 1, severe aortic and mitral regurgitation in 1, mitral and aortic valve endocarditis in 1, aorto-tracheal pseudoaneurysm in 1, and a fistula between the aortic root and right atrium in 1. No patient underwent reintervention for disease of the descending aorta. The surgical procedures applied are shown in Table 6. Hospital mortality was 23% (3/13), 2 patients died due to intraoperative bleeding and 1 due to low cardiac output.
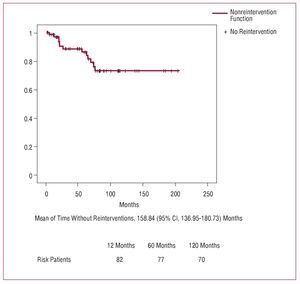
After 1, 5, and 10 years, respectively, 98.6% (1.3%), 86.2% (4.6%), and 68.2% (8.9%) of the surviving patients (Figure 2) did not need reintervention. The variables included in the univariate analysis are shown in Table 7. The Cox regression analysis indicates that severe preoperative aortic regurgitation (RR=3.3; P=.024) and sparing the aortic valve (RR=4.7; P=.05) were independent predictors of reintervention.
Figure 2. Nonreintervention curve
Long-Term Survival
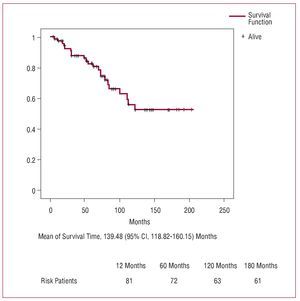
Total mortality was 27% (22/83). After 1, 5, 10, and 15 years, respectively, overall actuarial survival (Figure 3) was 82.4% (3.9%), 69.6% (5.4%), 47.3% (7%), and 44.1% (7.2%), and among hospital survivors this was 97.2% (1.9%), 82.5% (4.8%), 55.9% (7.9%), and 52.3% (8.2%). The causes of mortality during follow-up are shown in Table 8.
Figure 3. Survival curve during follow-up (Kaplan-Meier).
The univariate analysis showed old age (≥70 years), the inclusion technique, postoperative TND, and the application of non-antegrade cerebral protection methods (Table 9) as predictors of mortality during follow-up. In the multivariate analysis, the only statistically significant independent predictor of mortality during follow-up was not using antegrade cerebral protection (RR=3.1; P=.02).
DISCUSSION
Acute type-A aortic dissection is a critical situation that requires immediate clinical response and emergency surgical intervention in most cases.
Siegal et al18 estimated that, in untreated patients, the risk of mortality increases by 1% every hour during the first 48 h and reaches 70% after 1 week. According to data from the International Registry of Acute Aortic Syndrome (IRAD),1 mortality among patients treated conservatively is 58% versus total surgical mortality is 24%.
Although in recent years great progress has been made in diagnostic and surgical techniques and postoperative care, there is considerable variability in hospital mortality, ranging between 15 and 30%.2,3,19-21 In our experience, hospital mortality was 15%, similar to the best results of current series.4,22
The predictors of hospital mortality coincide with those of most of the published series.2-4,21 According to data from the IRAD,1 the independent variables of mortality are advanced age, hypotension/shock, previous heart disease, and preoperative renal, mesenteric or myocardial ischemia. These results show that hospital mortality is usually associated with the preoperative clinical characteristics of the patients and that are difficult to modify. Rampoldi et al1 have shown that unstable patients have a surgical mortality rate that is at least double that of stable patients (31.4% vs 16.7%). These findings reveal the importance of preoperative hemodynamic stability and emphasize the need for emergency surgery before the onset of hemodynamic deterioration.
In our series, the only independent predictors of hospital mortality were old age (≥70 years) and preoperative cardiogenic shock.
The extent of the surgical resection was not identified as an independent risk factor of hospital mortality; more extensive resections with replacement of the aortic arch did not affect the early or late results. Other authors, on the other hand, have proposed the exclusive resection of the ascending aorta, arguing that the risk involved in more extensive resections outweighs the potential benefit and that the main aim of surgery is patient survival.5
The cause of neurological injury during surgery for aortic dissection is multifactorial, and can lead to prolonged circulatory arrest, embolic events and poor cerebral perfusion due to preferential flow through the false lumen.
Postoperative neurological complications are a frequent cause of morbidity and mortality in aortic dissection surgery, with an estimated incidence of 10%-20%.3,5,7,8
Ergin et al9 and Hagl et al10 point out that postoperative stroke mainly causes embolic events in a possible association with retrograde perfusion from the femoral artery, but not directly with the cerebral protection method used, whereas TND would be associated with inadequate cerebral protection. Antegrade cerebral perfusion has been associated with a significant reduction in TND in recent series,10,11,23,24 although its role is less clear in relation to reducing stroke.
Antegrade cerebral perfusion has modified the concept of circulatory arrest in these patients because, strictly speaking, total circulatory arrest is not performed, given that continuous cerebral perfusion is maintained between 500 mL/min and 1000 mL/min. Furthermore, several authors have proposed that moderate hypothermia (25oC) would be sufficient to protect the brain and avoid the harmful effects of profound hypothermia.17,25,26 However, our group applied profound hypothermia as these repairs are complex, expected to be of long duration, and because this method of cooling has been shown to be very effective in protecting the brain as well as the other organs.17,27,28
Axillary artery cannulation, instead femoral, reduces the risk of poor visceral and cerebral perfusion, remobilization of thrombi from the abdominal and thoracic aorta toward the brain and, in addition, redirects flow to the true lumen, decreases the chance of excessive pressure build-up when clamping the aorta and facilitates the restoration of antegrade distal perfusion.12-16 All these advantages seem to indicate that antegrade cerebral perfusion via the axillary artery, except when this is affected by severe atherosclerosis, is the method of choice for cerebral protection.10,11,17
In our series, antegrade cerebral perfusion using the axillary artery reduced the incidence of TND to 6% and hospital mortality to 6% in the last 16 patients who underwent surgery, suggesting, but not demonstrating, its role in protecting against the neurological complications and their concomitant morbidity and mortality. Possibly, this lack of statistical significance is due to the low number of interventions using antegrade perfusion via the axillary artery. Given the rarity of this intervention (<7 patients per year), several years may still be needed before evidence can be obtained on the protective effect of this type of perfusion, and thus multicenter studies, such as the IRAD, are of great use in relation to these uncommon disorders.
At 10 years, 73.3% of patients had not undergone reintervention and this outcome is similar to those published.3,4,6,29,30 The aortic valve was spared whenever possible and only in cases of valvular degeneration, annuloaortic ectasia or previous valvular heart disease was it decided to perform valve replacement alone or with aortic root replacement (Bentall technique), a procedure that did not modify hospital mortality.
In our series, 77% of patients who underwent reintervention (13/78) required this due to severe aortic regurgitation that had previously presented and been conservatively treated by resuspension of the valve during the initial intervention. Preoperative acute aortic regurgitation and sparing the aortic valve were precisely the factors determining late aortic reintervention, and this would justify aggressive management of the aortic valve during the initial procedure.6,29 Hagl et al31 recommended systematic replacement of the root and aortic valve (Bentall technique). On the other hand, Estrera et al32 reported an acceptable level of durability after sparing the aortic valve; they pointed out that a possible reintervention should not dictate the initial procedure and recommended sparing the aortic valve to avoid chronic anticoagulant therapy and its possible role in the absence of thrombosis of the false lumen.33
We consider that the initial presentation of severe aortic regurgitation determines greater severity of the dissection, whose origin or extent includes the aortic root, and thus aggressive management with aortic root and valve resection and valve graft implantation (Bentall technique) should be the procedure of choice.4,6,29,31 In these cases, valve resuspension, by sparing the aortic root, leaves behind an intrinsically diseased segment of the aorta that presents greater risk of redissection and subsequent complications. The results published on the aortic root replacement technique with aortic valve reimplantation (David technique) are promising and, although long-term assessment is needed, it could become an interesting option.33,34
Of the 56 patients surviving hospital discharge who underwent CT during follow-up, only in 6 (11%) was progressive dilatation in other regions of the aorta evident with surgical indication: 2 patients with abdominal aorta aneurysm, 2 with thoracoabdominal aneurysm, and 2 with descending thoracic aorta aneurysm. Of these patients, 2 refused reintervention and the others had some comorbidity (advanced age, severe neurological deficit, etc) that made the procedure impossible.
Such a low rate of reintervention of the distal aorta is probably due to the high percentage of primary intimal tears located during the intervention and our decision to resect it in all cases. In fact, of the 15 patients in whom the intimal tear was not found, 60% (9 patients) were members of the group that did not undergo circulatory arrest (which prevents examination of the aortic arch and rules out intimal tears at that level) and that presented an increased risk of reintervention close to the limits of statistical significance (P=.06). Thus, several authors describe the lack of resection of the intimal rupture during the initial surgery as the main factor involved in late reintervention for dilatation of the thoracic or abdominal aorta.6,30
Although our group does not have experience in this regard, some authors point out that in given cases the implantation of a stent in the descending thoracic aorta during the initial procedure or a second could improve the outcomes by decreasing the incidence of reintervention and the onset of the complications due to the disease progressing to the distal aorta.35,36
The actuarial survival curve after hospital discharge indicates some percentages comparable to the ones described by other authors.2,3,5,6 Old age is one of the independent risk factors of mortality during follow-up in most series, partly due to the low life-expectancy in this group of patients.2 In general, there is a great deal of variability in determining the independent predictors of long-term survival. Chiappini et al2 described a series of 487 patients who underwent intervention for type-A aortic dissection and found that the only risk factor was preoperative diabetes. Erwin et al,3 in a group of 315 patients, identified advanced age and postoperative dialysis as the predictors of mortality during follow-up.
In our series, we found that not using antegrade cerebral protection was a predictor of late mortality, indicating that, although antegrade perfusion did not significantly modify the initial postoperative results, it has great importance regarding late survival.
Ergin et al9 associated postoperative TND with long-term cerebral function impairment. Pompilio et al37 assessed the influence of perioperative neurological events on late mortality, that is, the patients who survived hospital stay due to neurological injury had worse long-term survival rates. This fact may be explained by the high risk of bronchopneumonia, new neurological events and other complications related to the reduced functional capacity of these patients.4
Limitations
This article shares all the limitations associated with retrospective nonrandomized studies. The low incidence of this disease means that few patients underwent intervention, thus limiting the statistical power of the findings.
As this review covers a long period of experience, the first patients, who were managed without circulatory arrest, and the later patients, who were managed with antegrade cerebral perfusion, were not contemporary with the other cerebral protection methods mentioned. The last method (antegrade cerebral perfusion) was applied to a small sample size (16 patients), limiting the statistical significance of the results.
CONCLUSIONS
Acute aortic dissection surgery yields acceptable short-and long-term results and emergency intervention should be indicated in most patients.
Severe aortic regurgitation is associated with a high risk of early reintervention and this would justify aggressive management of the aortic valve during initial surgery.
Antegrade cerebral perfusion has modified the concept of circulatory arrest and has improved long-term prognosis among these patients. We consider that, although experience with more extensive series is required, the application of antegrade cerebral perfusion in acute aortic dissection surgery could improve prognosis among these patients.
ABBREVIATIONS
CT: computerized tomography
TND: temporary neurological dysfunction
Correspondence: Dr. C.E. Martín.
La Alcazaba, 2, 3.o D. 28041 Madrid. España.
E-mail: carlosestebanmartin@hotmail.com
Received November 23, 2007.
Accepted for publication June 10, 2008.