There are only a few anecdotic reports of aortic infective endocarditis (IE) treated with transcatheter aortic valve replacement (TAVR).1,2 Although dysfunction of a damaged valve can be treated with a TAVR device, persistent local infection requires debridement of the affected tissue and precludes the use of TAVR since reinfection would carry a dreadful prognosis.2 Thereafter, IE has been an exclusion criterion in most landmark studies and the use of TAVR in this context has been empirically disregarded. In contrast, it is well known that antibiotic treatment in IE is highly effective in some particular etiologies and, often, the only reason for cardiac surgery is the residual symptomatic severe valvular dysfunction.3 On this basis, TAVR might represent a novel alternative in this particular high operative risk subset if specific markers of healed infection could be determined.
The aim of this study was to identify the main predictors of active local infection at the time of intervention that would preclude TAVR use in IE. Among a total of 732 episodes of left-sided IE consecutively diagnosed in 2 tertiary centers between 1996 and 2015, 432 patients underwent cardiac surgery and 224 of them had involvement of either native or biological prosthetic aortic valves. Only patients with culture of the removed cardiac tissue (n = 182) were included. In addition, patients with discordant positive valve culture (n = 14) were excluded due to the impossibility of ruling out culture contamination.
We defined active local infection at the time of intervention as the presence of either periannular complications or concordant positive cultures (same microorganism in the blood and the cardiac tissue removed during surgery). Biological tissues were grown on brain heart broth and thioglycollate, and on 4 types of agar media (Columbia sheep blood, chocolate supplemented with IsoVitaleX, McKonkey, and Schaedler).
To determine predictors of active local infection at the time of intervention, we built a predictive model using a logistic regression model with the maximum likelihood method and backward stepwise selection, which included the variables that were clinically relevant and statistically significant in the univariable analysis. Only the last step is shown. The goodness-of-fit for each model was determined with the Hosmer–Lemershow test and the area under the receiver operating characteristics curve (AUC-ROC).
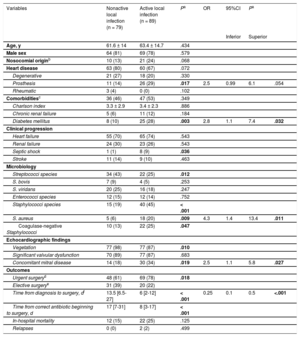
The Table summarizes the univariable and multivariable predictors of active local infection at the time of intervention. The main independent predictors of active local infection were diabetes mellitus (odds ratio [OR], 2.8; 95% confidence interval [95%CI], 1.1-7.4), Staphylococcus aureus (OR, 4.3; 95%CI, 1.4-13.4) and concomitant mitral involvement (OR, 2.5; 95%CI, 1.1-5.8). In contrast, an interval between diagnosis and intervention ≥ 10 days (estimated cut-off value) was a predictive factor of healed infection (OR, 0.25; 95%CI, 0.1-0.5). The model had an AUC-ROC of 0.776 (95%CI, 0.705-0.847) and a Hosmer–Lemershow P value of .848. Indeed, after 10 days of appropriate antibiotic treatment and in the absence of diabetes mellitus, Staphylococcus aureus, concomitant mitral involvement, or aortic prosthesis, only 1 patient out of 29 (3.5%) had a positive culture at the time of intervention.
Univariable and Multivariable Predictors of Active Local Infection at the Time of Cardiac Surgery in Patients With Aortic Valve Infective Endocarditis
| Variables | Nonactive local infection (n = 79) | Active local infection (n = 89) | Pa | OR | 95%CI | Pa | |
|---|---|---|---|---|---|---|---|
| Inferior | Superior | ||||||
| Age, y | 61.6 ± 14 | 63.4 ± 14.7 | .434 | ||||
| Male sex | 64 (81) | 69 (78) | .579 | ||||
| Nosocomial originb | 10 (13) | 21 (24) | .068 | ||||
| Heart disease | 63 (80) | 60 (67) | .072 | ||||
| Degenerative | 21 (27) | 18 (20) | .330 | ||||
| Prosthesis | 11 (14) | 26 (29) | .017 | 2.5 | 0.99 | 6.1 | .054 |
| Rheumatic | 3 (4) | 0 (0) | .102 | ||||
| Comorbiditiesc | 36 (46) | 47 (53) | .349 | ||||
| Charlson index | 3.3 ± 2.9 | 3.4 ± 2.3 | .886 | ||||
| Chronic renal failure | 5 (6) | 11 (12) | .184 | ||||
| Diabetes mellitus | 8 (10) | 25 (28) | .003 | 2.8 | 1.1 | 7.4 | .032 |
| Clinical progression | |||||||
| Heart failure | 55 (70) | 65 (74) | .543 | ||||
| Renal failure | 24 (30) | 23 (26) | .543 | ||||
| Septic shock | 1 (1) | 8 (9) | .036 | ||||
| Stroke | 11 (14) | 9 (10) | .463 | ||||
| Microbiology | |||||||
| Streptococci species | 34 (43) | 22 (25) | .012 | ||||
| S. bovis | 7 (9) | 4 (5) | .253 | ||||
| S. viridans | 20 (25) | 16 (18) | .247 | ||||
| Enterococci species | 12 (15) | 12 (14) | .752 | ||||
| Staphylococci species | 15 (19) | 40 (45) | < .001 | ||||
| S. aureus | 5 (6) | 18 (20) | .009 | 4.3 | 1.4 | 13.4 | .011 |
| Coagulase-negative Staphylococci | 10 (13) | 22 (25) | .047 | ||||
| Echocardiographic findings | |||||||
| Vegetation | 77 (98) | 77 (87) | .010 | ||||
| Significant valvular dysfunction | 70 (89) | 77 (87) | .683 | ||||
| Concomitant mitral disease | 14 (18) | 30 (34) | .019 | 2.5 | 1.1 | 5.8 | .027 |
| Outcomes | |||||||
| Urgent surgeryd | 48 (61) | 69 (78) | .018 | ||||
| Elective surgerye | 31 (39) | 20 (22) | |||||
| Time from diagnosis to surgery, df | 13.5 [6.5-27] | 6 [2-12] | < .001 | 0.25 | 0.1 | 0.5 | <.001 |
| Time from correct antibiotic beginning to surgery, d | 17 [7-31] | 8 [3-17] | < .001 | ||||
| In-hospital mortality | 12 (15) | 22 (25) | .125 | ||||
| Relapses | 0 (0) | 2 (2) | .499 | ||||
95%CI: 95% confidence interval; OR: odds ratio.
The data are expressed as mean ± standard deviation or median [interquartile range] or No. (%).
Nosocomial origin: signs and symptoms of infective endocarditis starting after 48hours from hospital admission or in the first 3 days after discharge or up to 30 days after a surgical intervention.
Comorbidities: defined by the presence of either diabetes mellitus, chronic renal failure, immunosuppression, chronic pulmonary disease, cancer, collagenopathy requiring steroids, HIV or intravenous drug use.
Recommendations against the use of TAVR in the context of uncomplicated aortic valve IE are based on unfounded but extensively accepted arguments. For the first time, we have evaluated the actual risk of this potential management in a large population of surgical patients whose resected tissue was cultured, demonstrating that most patients have a predictable lack of local infection after antibiotic therapy. This hypothesis-generating finding might support the use of TAVR in selected cases of IE with “healed” infection but residual lesion and high surgical risk. Conversely, periannular complications, the need for extensive surgical repair, septic shock, and infection of biological prosthesis might be related to persistent infection, suggesting that TAVR should be also avoided in these scenarios until further data are available.4,5
In conclusion, our findings suggest that in poor surgical candidates and under the assessment of a multidisciplinary experienced IE team, TAVR could be considered as an alternative therapeutic option in selected cases of IE with low risk of local infection at the time of the planned intervention.
.